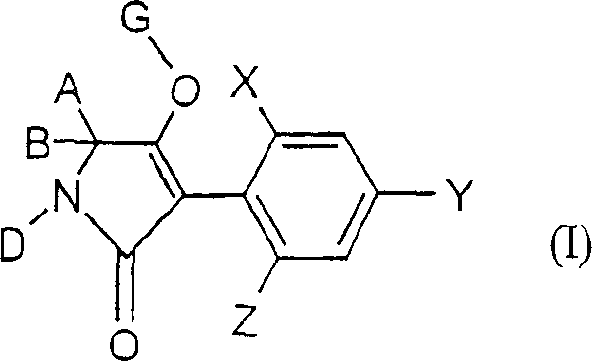
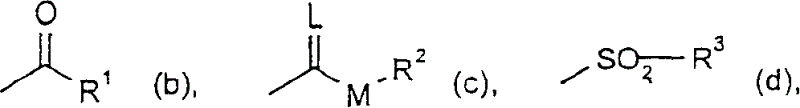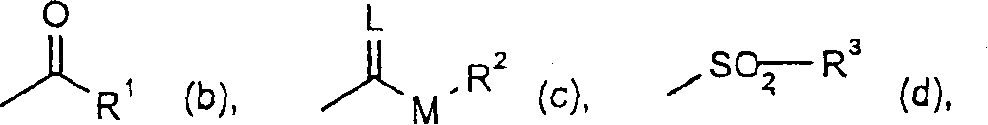2,4-dihalogen-6-(c2-c3-alkyl)-phenyl substituted tetramic acid derivatives
A halogenated alkyl, alkyl technology, applied in the field of 2,4-dihalogen-6-(C2-C3-alkyl) phenyl substituted tetramic acid derivatives, can solve the compatibility is not always sufficient, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I-a-1
[0867]
[0868] At 0 to 20°C, add 5.03g of the compound of formula II-1 in 10ml anhydrous dimethylformamide solution to 2.92g (0.023mol) potassium tert-butoxide in 8ml anhydrous dimethylformamide solution, and The resulting mixture was stirred at 20°C.
[0869] The reaction solution was poured into 80 ml of ice water, and then the pH of the solution was adjusted to 1 with concentrated hydrochloric acid at 0-20°C, the precipitate was filtered off with suction and dried. Then the product was triturated with MTB ether / n-hexane.
[0870] Yield: 3.79 g (80% of theory), melting point: 245°C.
[0871] Similar to Example (I-a-1) and according to its general preparation method, the following compound of formula (I-a) was obtained:
[0872]
[0873] Example
serial number
X
Y
Z
D
A
B
Fp.℃
isomer
I-a-2
Cl
Cl
C 2 H 5
H
-(CH 2 ) 2 -CHCH 3 -(CH 2 ) 2 -
>220
β
...
Embodiment I-b-1
[0881]
[0882] 0.5ml (3.6mmol) of triethylamine was added to 1.3g of the compound of Example I-a-1 in 30ml of anhydrous ethyl acetate. A solution of 0.38 ml (0.0036 mmol) of isobutyryl chloride in 5 ml of anhydrous ethyl acetate was added dropwise under reflux.
[0883] The mixture was stirred at reflux. The completion of the reaction was detected by thin layer chromatography. The solvent was removed with a rotary evaporator, the residue was dissolved in dichloromethane and washed twice with 50 ml of 0.5N NaOH solution, dried, and then the solvent was evaporated. The product was then recrystallized from MTB ether / n-hexane.
[0884] Yield: 0.81 g (55% of theory), melting point: 155°C.
[0885] Similar to Example (I-b-1) and according to its general preparation method, the following compound of formula (I-b) was obtained:
[0886]
[0887] Example
serial number
X
Y
Z
D
A
B
R 1
Fp.℃
isomer
...
Embodiment I-c-1
[0897]
[0898] At 0-10°C, a solution of 0.6ml (0.006mol) ethyl chloroformate in 50ml of dry dichloromethane was added dropwise to 2.34g of the compound of Preparation Example Ia-14 in 50ml of dry dichloromethane and 0.84ml 6mmol) in triethylamine. The mixture was stirred at room temperature until the reaction was complete (checked by thin layer chromatography).
[0899] Then the solvent was evaporated, the residue was dissolved in dichloromethane and washed twice with 50 ml of 0.5N NaOH solution, dried, then the solvent was evaporated, and the residue was recrystallized from MTB ether / n-hexane.
[0900] Yield: 2.2 g (79% of theory), melting point: 114°C.
[0901] Similar to Example (I-c-1) and according to its general preparation method, the following compound of formula (I-c) was obtained:
[0902]
[0903]
[0904] Example
serial number
X
Y
Z
D
A
B
M
R 2
Fp.℃
isomer
I-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com