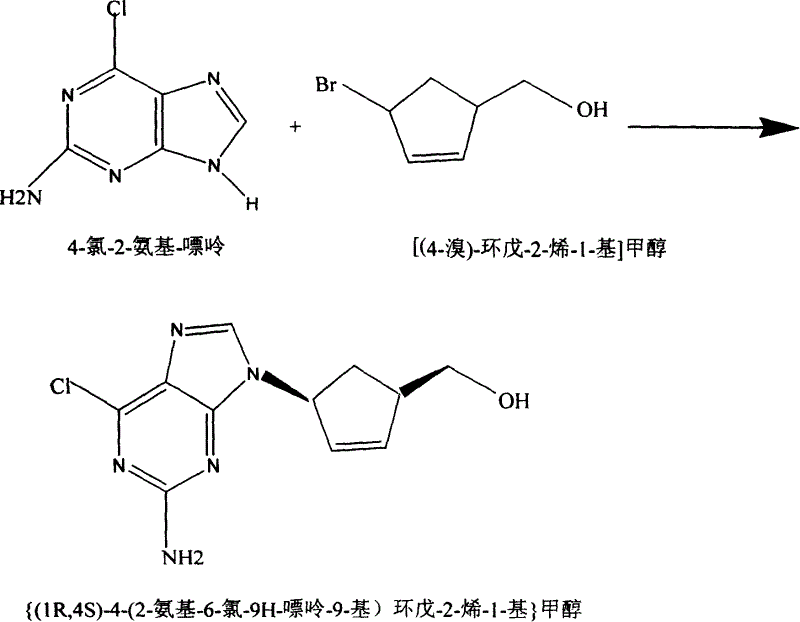
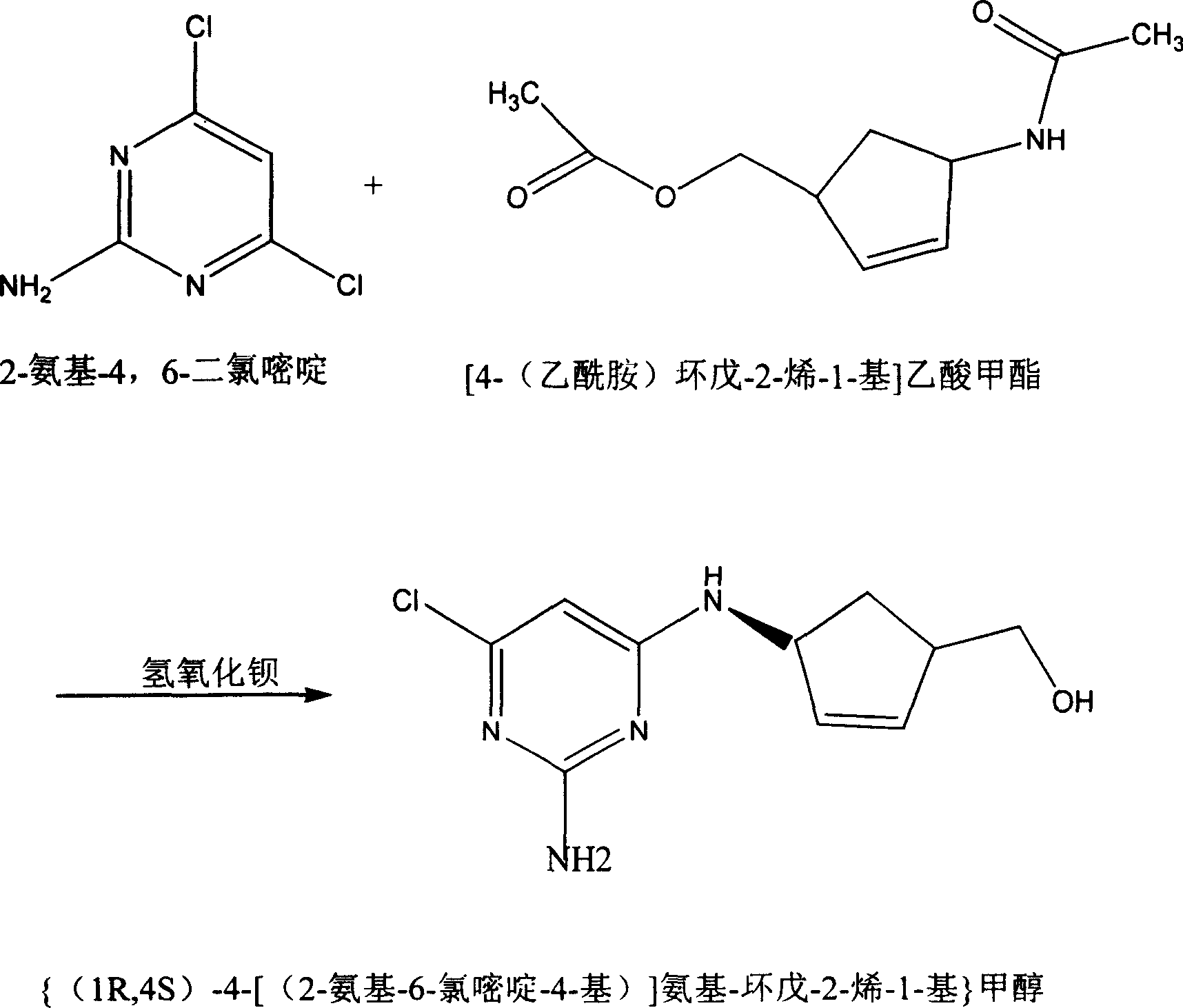
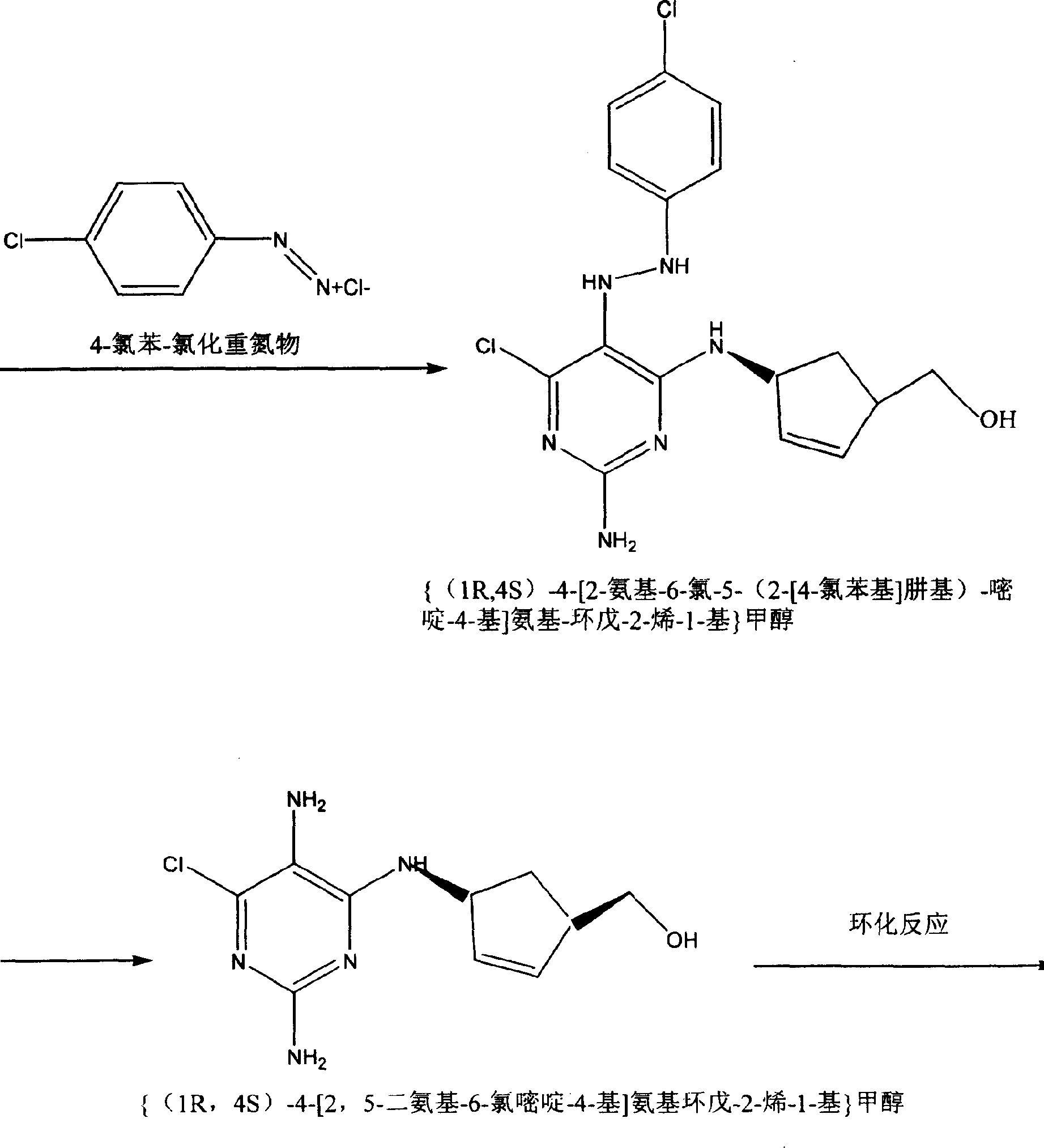
Abacavin preparing process
A technology of amino and methanol, applied in organic chemistry, antiviral agents, etc., can solve problems such as cumbersome processes, achieve the effects of easy-to-obtain raw materials, save steps, and reduce the cost of finished medicines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0015] A: Synthesis of [(4-bromo)-cyclopent-2-en-1-yl]methanol
[0016] Add 11.3 grams (0.1 mol) of [(4-amino)-cyclopent-2-en-1-yl]methanol (synthesized according to relevant patents) into a 250 ml four-neck bottle, cool, stir, and slowly add 50 ml 48% hydrobromic acid, cooled to about 0°C. Slowly add 40 grams of aqueous solution made of 6.9 grams (0.1 mole) of sodium nitrite dropwise. Keep the reaction temperature in the bottle at about 0°C, and use starch-potassium iodide test paper to detect the end point of diazotization before the drop is completed. The diazonium reaction solution after the end point was still placed in ice salt.
[0017] In a 500ml four-necked flask equipped with a condenser, a stirrer and a thermometer, 10 grams of cuprous bromide, 20 grams of 48% hydrobromic acid and 0.5 gram of phenothiazine (polymerization inhibition) were added. Heat to near boiling. Under vigorous stirring, the heating was continued, and the above diazotization reaction solutio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com