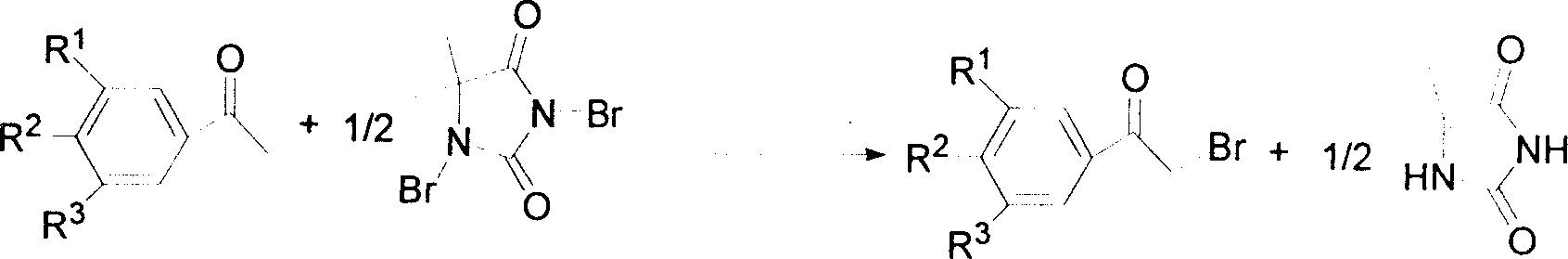
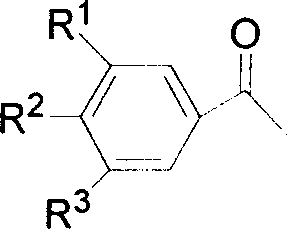
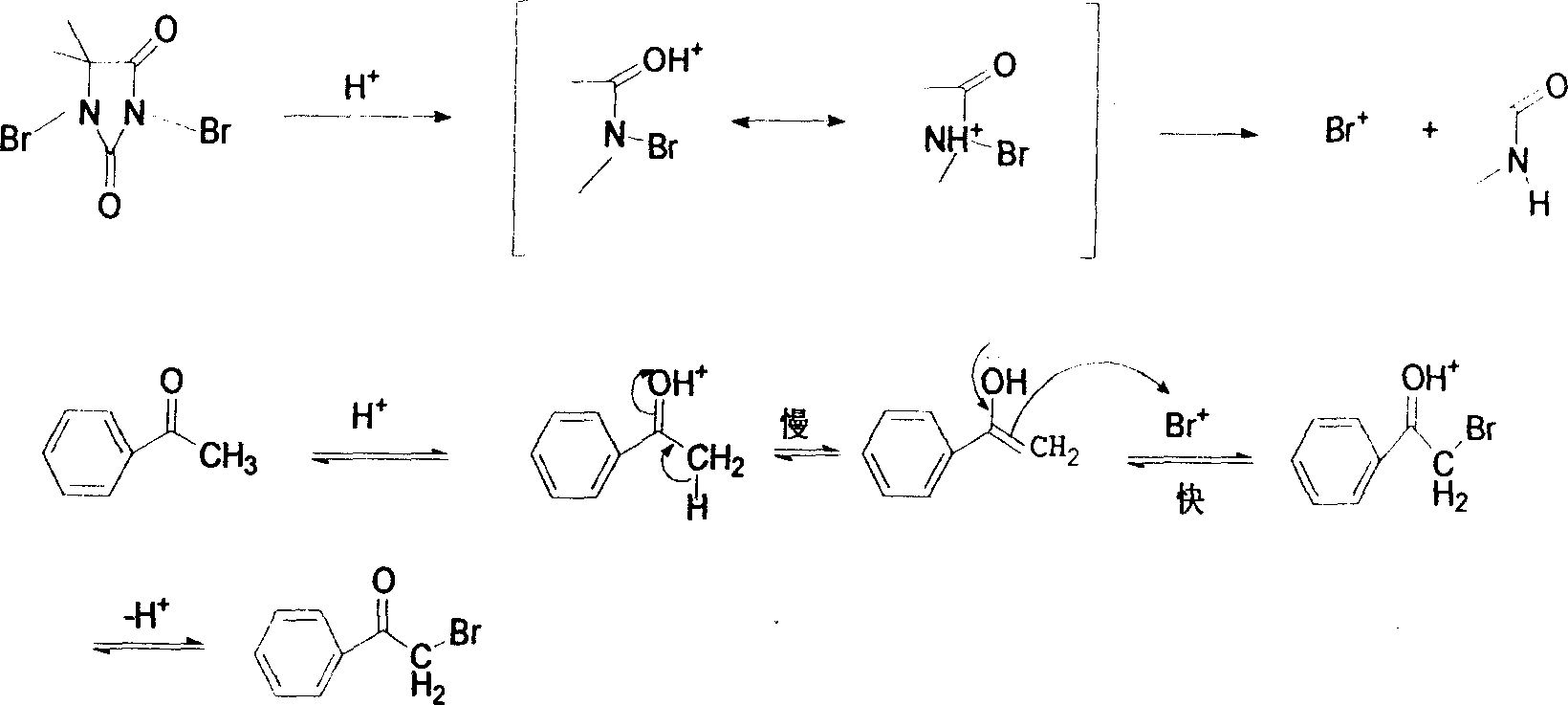
Method for synthesizing alpha-bromo-acetophenone
A technology of bromoacetophenone and acetophenone, which is applied in the field of α-bromination to replace acetophenone, can solve the problems of unused cost, low product purity, and pungent odor, and achieve easy The effect of industrialized production and laboratory operation, simple process and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Add 8.6mmol of acetophenone, 4.3mmol of p-toluenesulfonic acid, 15mL of methanol into a 100mL three-neck flask, and stir at 20°C. Add dropwise 6.4mmol of DBDMH in 25mL of methanol solution (dissolve 6.4mmol of 1,3-dibromo-5,5-dimethylhydantoin in 25ml of methanol solvent to prepare a DBDMH solution, which is convenient for dropwise addition). The reaction was completed in 6 hours. After the reaction, methanol was removed by rotary evaporation, and then about 80 mL of ice water was added for shaking. Solids were precipitated, filtered by suction, and vacuum-dried at room temperature to obtain white crystals, which were the product α-bromoacetophenone of the present invention. The yield was 88%, melting point 48-50°C (48-51°C in literature). Add sodium carbonate to the filtrate to neutralize to neutral, remove water under reduced pressure, wash the residue with acetone, filter, remove solvent, and recover hydantoin.
Embodiment 2
[0021] Example 2: Add 10 mmol m-nitroacetophenone, 2 drops of phosphoric acid, and 15 mL toluene into a 100 mL three-necked flask, and stir at 60° C. Add 7.5mmol of DBDMH in 25mL of toluene solution dropwise (dissolve 7.5mmol of 1,3-dibromo-5,5-dimethylhydantoin in 25ml of methanol solvent to prepare a DBDMH solution, which is convenient for dropwise addition). The reaction was completed in 5 hours. After the reaction was completed, the methanol was removed by rotary evaporation, and then about 80 mL of ice water was added for shaking, and a solid was precipitated, filtered by suction, and dried in vacuo at room temperature to obtain white crystals with a yield of 74% and a melting point of 92-94 ° C (document: 90 -94°C). Add sodium carbonate to the filtrate to neutralize to neutral, remove water under reduced pressure, wash the residue with acetone, filter, remove solvent, and recover hydantoin.
[0022] The acetophenone raw material that other 22 embodiments adopt and the p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com