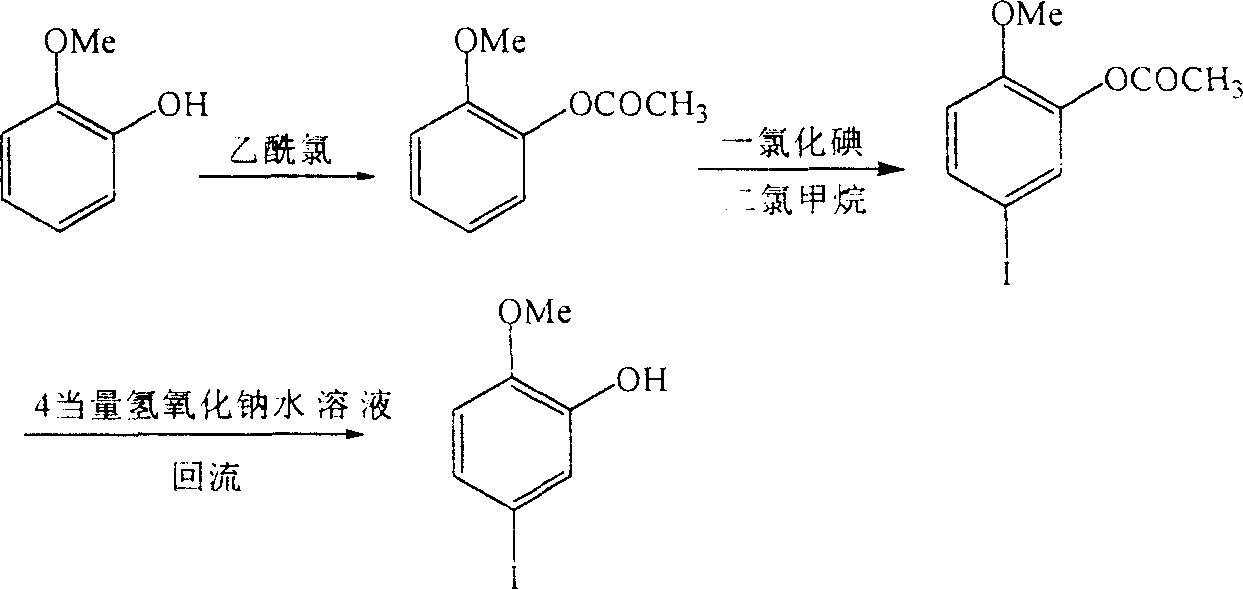
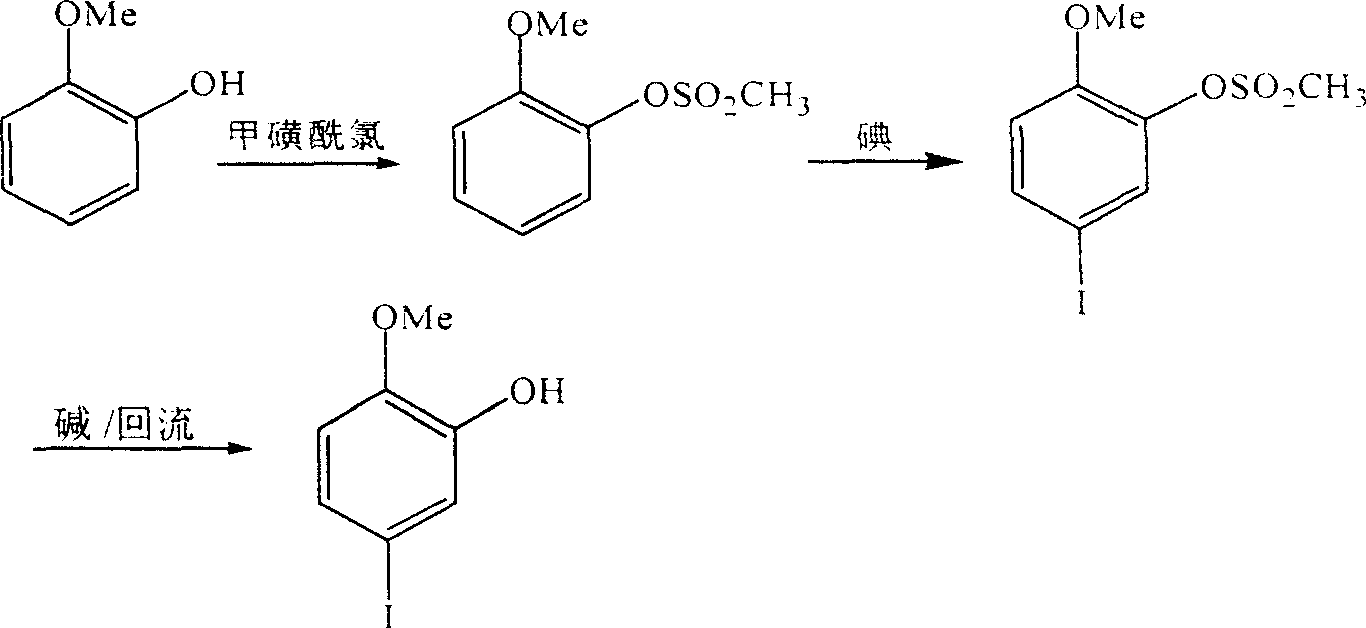
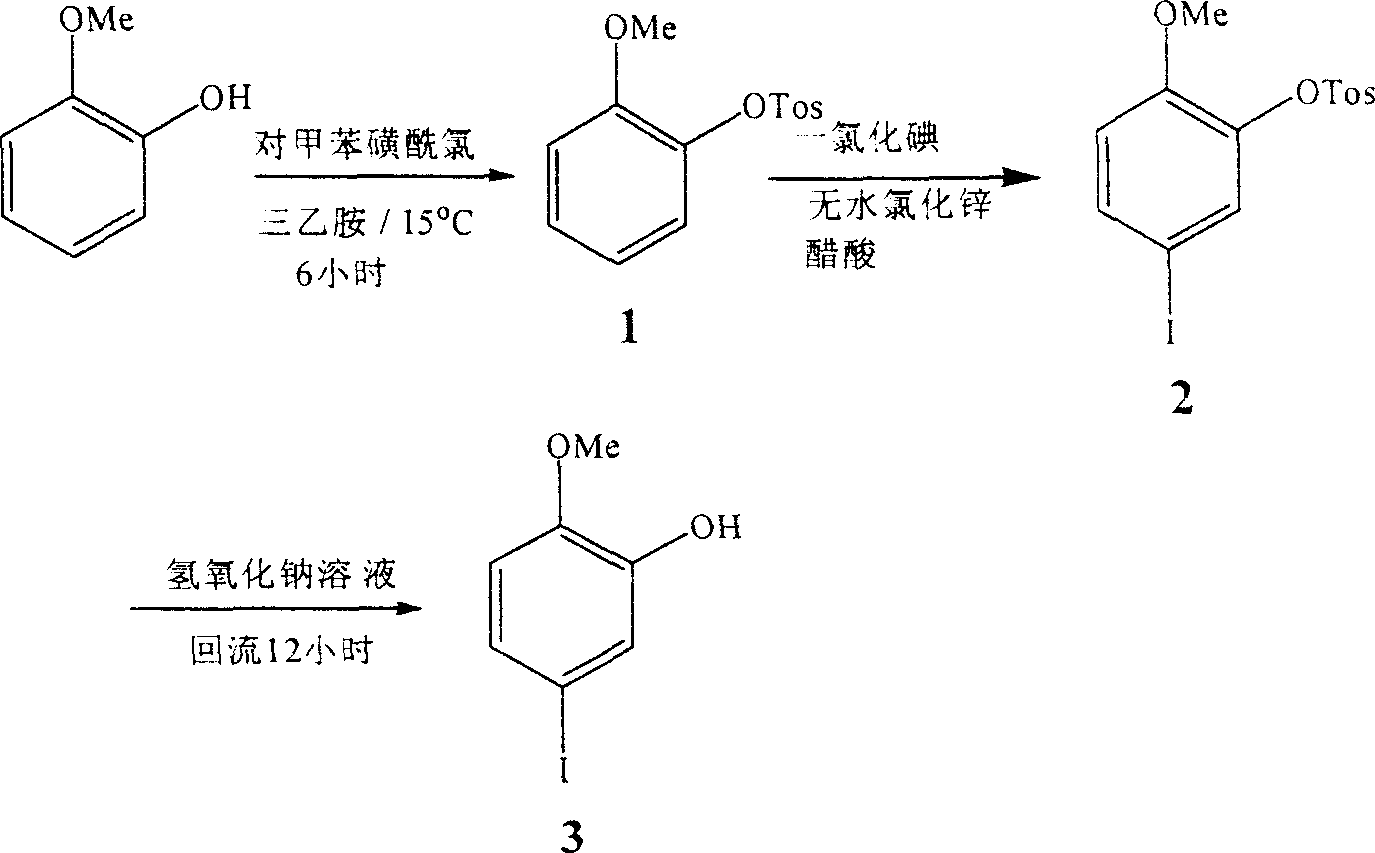
Synthesis of 2-methoxyl-5-iodophenol
A synthesis method, a methoxyl technology, applied in the direction of organic chemistry, etc., can solve the problems of poor separation effect, unsuitable for industrial production, expensive reagents, etc., and achieves few steps, positioning effect and easy separation of products, and cheap reagents. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment one: the specific steps of this embodiment are as follows:
[0020] 1. Synthesis of p-toluenesulfonic acid-(2-methoxy)phenol ester:
[0021] Take 30 grams of 2-methoxy-phenol and dissolve it in 300 ml of triethylamine. After stirring evenly, add an equivalent amount of p-toluenesulfonyl chloride, control the temperature below 15°C and continue stirring for 6 hours, and let it stand overnight. After filtering, the obtained solid was washed twice with triethylamine and water respectively to obtain 63 g of white solid with a yield of 93.3% and a melting point of 82.1-82.3°C. Infrared IR: 3003cm -1 , 1597cm -1 , 1499cm -1 , 1257cm -1 , 1185cm -1 , 867cm -1 , 781cm -1 , 758cm -1 . NMR 1 H-NMR (CDCl 3 )δ: 7.52 (dd, 4H), 7.16 (m, 2H), 6.88 (m, 2H), 3.55 (s, 3H), 2.44 (s, 3H).
[0022] 2. Synthesis of p-toluenesulfonic acid-(2-methoxy-5-iodo)phenol ester:
[0023] Weigh 33 grams of (2-methoxy)phenol p-toluenesulfonate, dissolve it in 200 milliliters of g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com