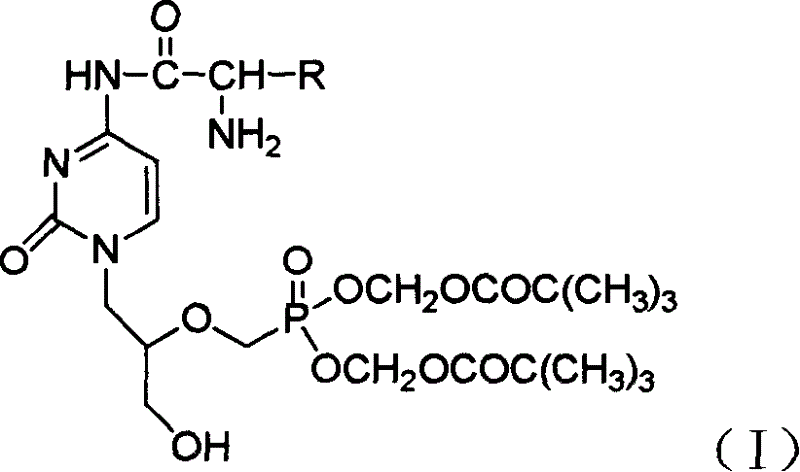
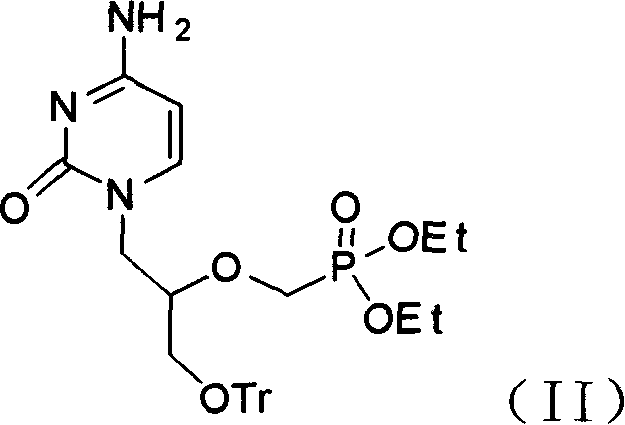
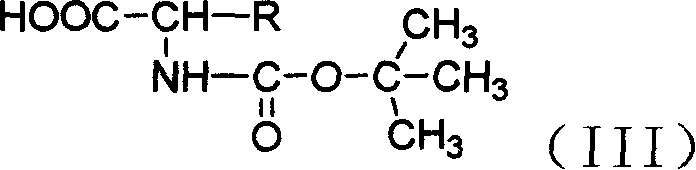
Antiviral agent cidofovir derivatives
A technology of derivatives and drugs, applied in the field of new derivatives of cidofovir, which can solve the problems of not enough to cause biological effects, difficult compatibility of lipophilicity, poor ability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Preparation of intermediate (R)-2,3-isopropylidene glycerol methanesulfonyl ester (5)
[0048] 1. Preparation of 1,2-5,6-bis(dioxyisopropylidene)mannitol (2)
[0049] Add 37.5g (0.206mol) of mannitol (1) to 40g (0.293mol) of zinc chloride and 250mL of acetone, stir at room temperature for 20-24h, dissolve 45g (0.325mol) of potassium carbonate in 45mL of hot water, and add dropwise to the reaction flask , stir for 15-20 minutes, filter, wash with 50ml×3 water, add about 15mL concentrated ammonia water to the filtrate, concentrate to obtain a white solid, add a small amount of water, extract 3 times with dichloromethane, dry with anhydrous sodium sulfate, and use it directly in the next step reaction. For example, the dichloromethane layer can be evaporated to dryness to obtain 51.75 g of white paste-like solid, the yield is 97-98% (the yield in the literature is 87%).
[0050] 1 H NMR (CDCl 3 ): δ 3.68 (dd, H-1, H-6), 3.27 (m, 4H, H-2, H-3, H-4, H-5)
[00...
Embodiment 2
[0060] Example 2 Preparation of intermediate benzoylcytosine (6)
[0061] 3g (0.027mol) cytosine was added to 300ml of pyridine, then 37.5mL of benzoyl chloride was added dropwise, stirred at room temperature for about half an hour, the dropping was completed, stirred for 45 minutes, added dropwise 2N hydrochloric acid, stirred at room temperature for 2h, suction filtered, the solid was 5% sodium hydroxide was dissolved, then 2N hydrochloric acid was added dropwise, the refrigerator was placed overnight, suction filtration, and vacuum drying to obtain 5.8 g of white solid, mp>300 ° C (document mp> 300 ° C), yield 95% (document yield is 89%).
[0062] 1 H NMR (d 6 -DMSO): δ 11.17 (s, 1H, NH), 11.27 (s, 1H, NH), 8.18 (d, J=7Hz, 1H, H-6), 7.96 (d, J=7Hz, 2H, 2 ×BzH), 7.85(d, J=6Hz, 1H, H-5), 7.76-7.46(m, 3H, 3×BzH)
Embodiment 3
[0063] Example 3 Intermediate (S)-N 1 Preparation of -[(3-Trityloxy-2-ethylphosphorylmethoxy)glycerol]-cytosine (11)
[0064] 1. (S)-N 1 -[2,3-O-Isopropylidene-2,3-(dihydroxy)glycerol]-N 4 - Preparation of Benzoylcytosine (7)
[0065] Method 1: finely ground benzoylcytosine (6) 6g (0.0276mol) and 5.4g (0.0390mol) finely ground potassium carbonate, add anhydrous N,N-dimethylformamide (DMF) 120mL, oil The bath was heated to 90°C, and 5.2g (0.0267mol) of (R)-2,3-isopropylidene glyceryl methanesulfonyl ester (5) dissolved in 50mL of DMF was slowly added dropwise. After about 1h, the reaction was complete. Suction filtration, wash the precipitate with DMF, concentrate the filtrate under reduced pressure, add 300 mL of dichloromethane to the obtained solid, stir for about 2 h, filter, wash the precipitate with dichloromethane, and concentrate the filtrate under reduced pressure to obtain a pale yellow oil, which is separated by column chromatography to obtain The white solid was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mp | aaaaa | aaaaa |
| Yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com