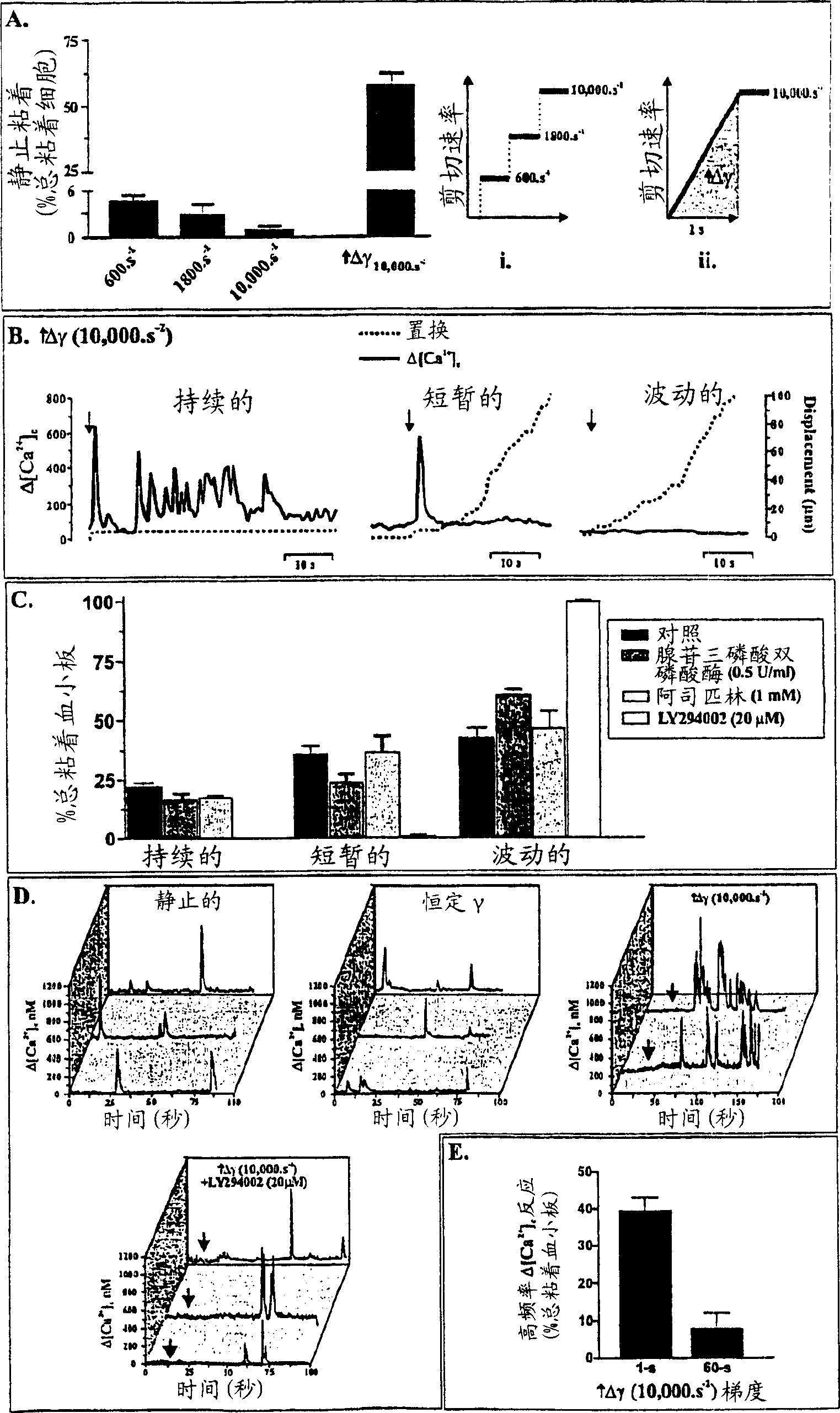
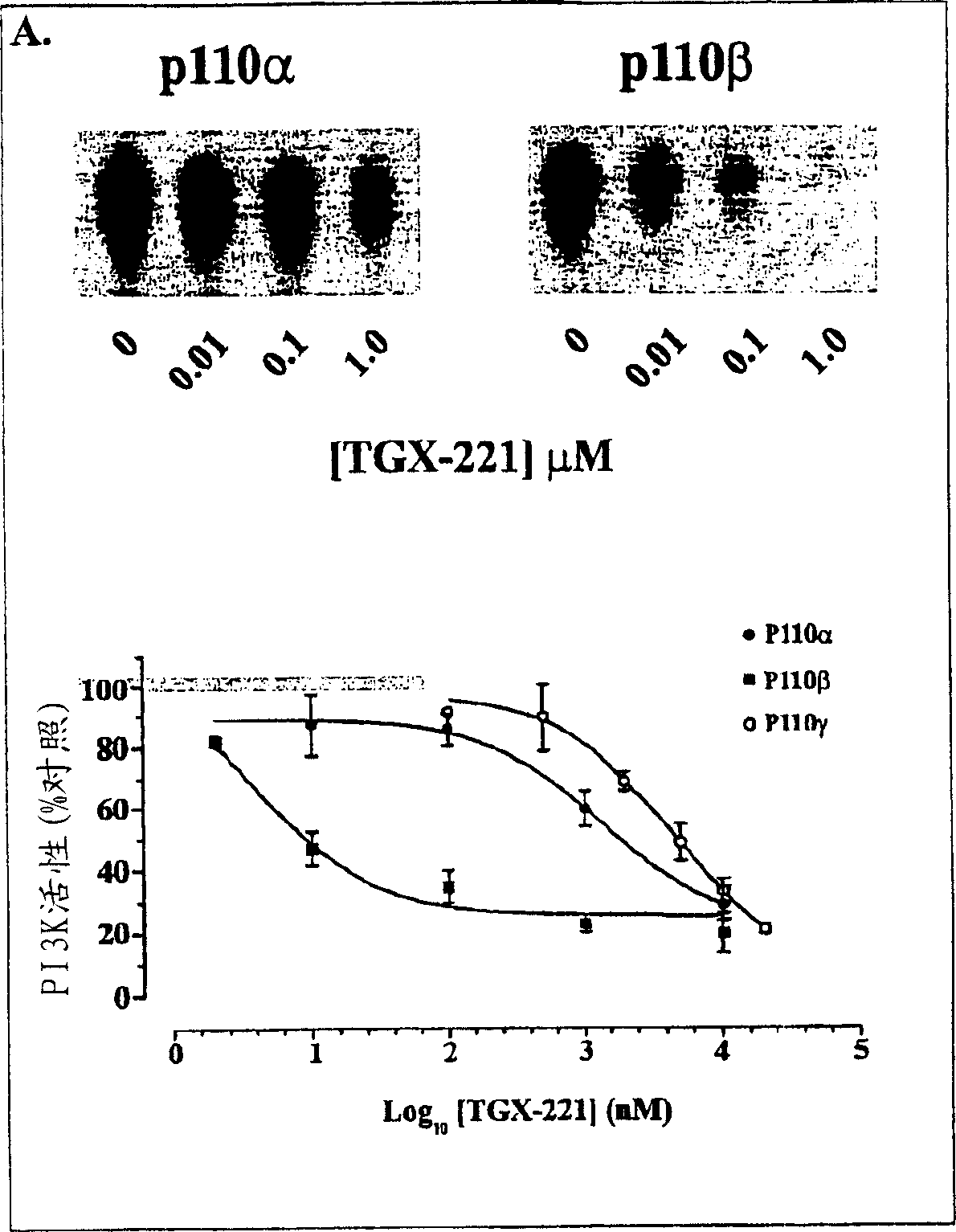
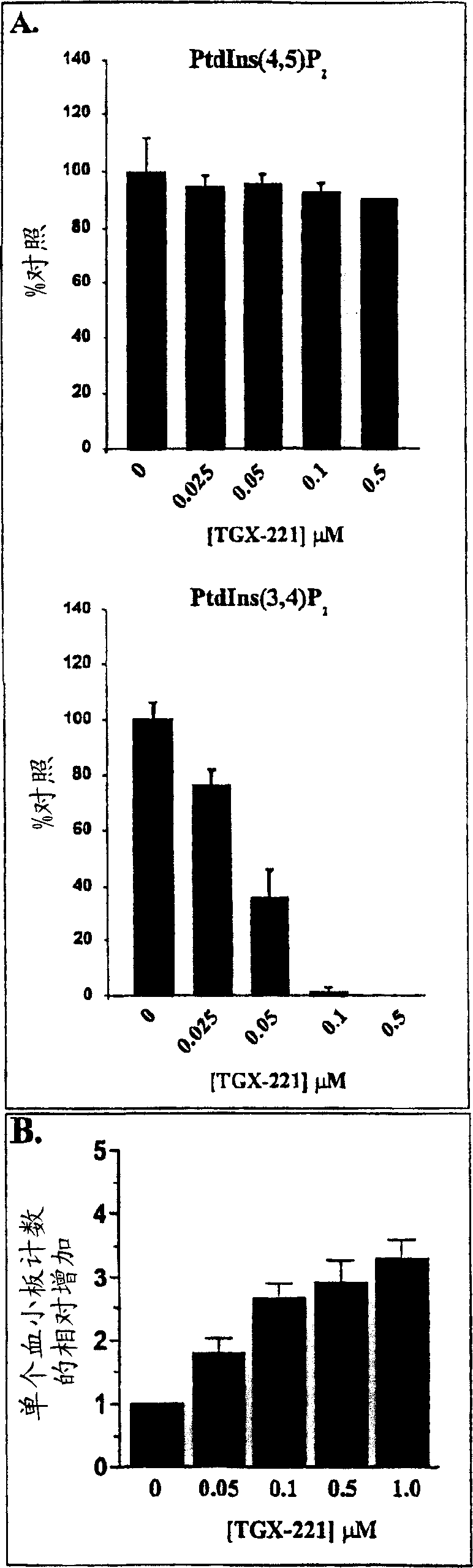
Inhibition of phosphoinositide 3-kinase beta
A technology of kinase and β inhibitors, applied in medical preparations containing active ingredients, blood diseases, extracellular fluid diseases, etc., can solve problems such as unidentified
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0208] Example 1. Synthesis of (±)-7-methyl-2-morpholin-4-yl-9-(1-phenylaminoethyl)-pyrido[1,2-a]pyrimidin-4-one ( TGX-221: R 1 =CH 3 , R 2 =CH 3 , R 3 =H)
[0209]
[0210] Compound 2: To a solution of 2-amino-3-bromo-5-methylpyridine (1) (45 g, 0.45 mol) in dichloromethane (500 mL) was added malonyl dichloride (25 mL , 0.25mol). The mixture was stirred at room temperature for 48 hours. The precipitated pale yellow solid was collected by filtration, washed with dichloromethane (3 x 100 mL), and dried under vacuum to give product 2 (52.5 g). The filtrate was concentrated under reduced pressure. The resulting residue was suspended in water and stirred for 1 hour. The solution was filtered, and the filtrate was washed with solid NaNCO 3 Neutralization gave unreacted 2-amino-3-bromo-5-methylpyridine (6 g). The crude compound was used in the next synthetic step without further purification.
[0211] 1 H NMR (300MHz, DMSO-d 6 )δ 8.72(s, 1H),
[0212] 8.28(s, 1H), ...
Embodiment 2
[0218] Example 2. Preparation of pyridine-substituted benzopyrone derivatives
[0219] 8-(Substituted)-2-(4-pyridyl)-4H-1-benzopyran-4-ones were prepared following the general procedure adapted from Cushman and Nagarathnam, 1990, Tetrahedron Letters 31:6497. Briefly, various precursors 2-hydroxyacetophenones (1) were treated with methyl isonicotinate and derivatives followed by ring closure and dehydration to yield pyridine-substituted products (3). Then through various coupling reactions in R 1 import specific substituents.
[0220]
[0221] Substituents on acetophenone (R) may include, but are not limited to, bromo, hydroxy, acetamido, methoxymethyl, methyl, ethyl, methoxy, trifluoromethanesulfonyloxy, and acetyl substitution base. Substituents on isonicotinate (R') include, but are not limited to, chloro, methyl and amino substituents. Reagents for the condensation reaction include lithium bis(trimethylsilyl)amide, sodium hydride, 1,8-diazabicyclo[2.2 .2] Undecane...
Embodiment 2a
[0225] Example 2a : 6-methyl-8-acetyl-2-(4-pyridyl)-4H-1-benzopyran-4-one
[0226]
[0227] 3′-Acetyl-2′-hydroxy-5′-methylacetophenone
[0228] A mixture of 2-hydroxy-5-methylacetophenone (15 g, 0.1 mol) in dichloromethane (100 ml) was treated with triethylamine (13.9 ml), dimethylaminopyridine (1.22 g) and acetic anhydride ( 9.5 ml) and stirred overnight at room temperature. The mixture was then poured into water (300ml) and extracted with dichloromethane (3 x 60ml). The combined mixture was washed with saturated NaHCO 3 Washed with aqueous solution, dried (Na 2 SO 4 ) and removal of the solvent gave a colorless oil (19.5 g).
[0229] The product was dissolved in dichloromethane (200ml) at 0°C and treated with aluminum chloride (19.5g), then stirred at room temperature for 5 days. The solution was treated with ice (50 g) and 2N hydrochloric acid (50 ml), then stirred at room temperature for 1 hour. The dichloromethane layer was separated and the aqueous layer wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com