Method for synthesizing organic sulfur compound under condition without solvent
A compound and carbonyl compound technology, applied in the field of environment-friendly synthesis, can solve the problems of harming the health of users, volatile penetration, inconvenient transportation, etc., and achieve the effect of mild reaction conditions, low price, and easy process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
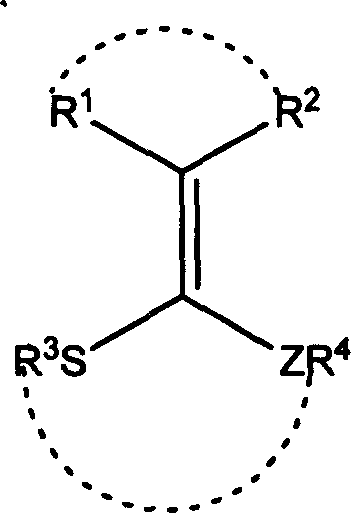
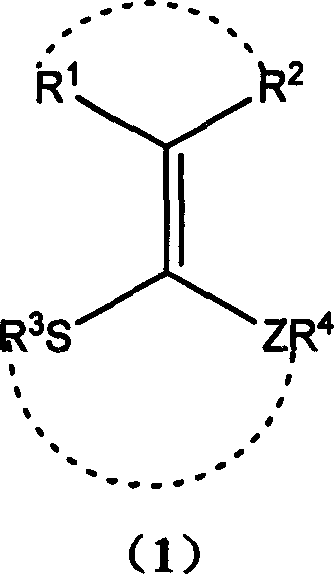
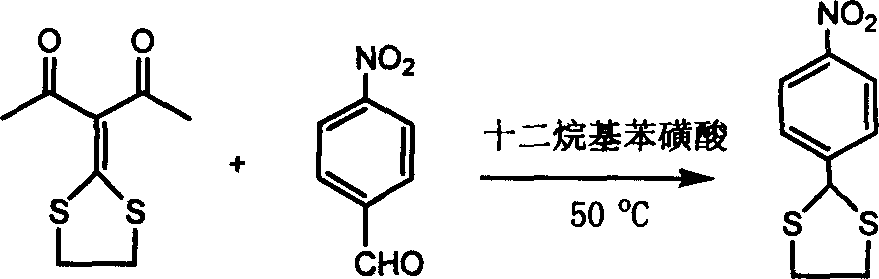
[0027] In a 50 ml round bottom flask, add 0.404 g (2 mmol) 3-(1,2-ethylenedithio)methylene-2,4-pentanedione, 0.302 g (2 mmol) 4- Nitrobenzaldehyde, 0.702 (2.15 mmol) grams of dodecylbenzenesulfonic acid, heated to 50 ° C, stirred for 45 minutes, TLC monitoring to the disappearance of 4-nitrobenzaldehyde, through column chromatography, a white solid 2- (4-nitrophenyl)-[1,3]dithiapentane 0.396 g, yield 87.2%. The reaction is shown in the following formula:
[0028]
Embodiment 2
[0030] In a 50 ml round bottom flask, add 0.404 g (2 mmol) 3-(1,2-ethylenedithio)methylene-2,4-pentanedione, 0.302 g (2 mmol) 4- Nitrobenzaldehyde, 0.702 g (2.15 mmol) dodecylbenzenesulfonic acid, stirred at 20°C for 150 minutes, monitored by TLC until 4-nitrobenzaldehyde disappeared, after column chromatography, a white solid was obtained to obtain 2- (4-nitrophenyl)-[1,3]dithiapentane 0.368 g, yield 81.1%. The reaction is shown in the following formula:
[0031]
Embodiment 3
[0033] In a 50 ml round bottom flask, add 0.404 g (2 mmol) 3-(1,2-ethylenedithio)methylene-2,4-pentanedione, 0.302 g (2 mmol) 4- Nitrobenzaldehyde, 0.702 g (2.15 mmol) dodecylbenzenesulfonic acid, heated to 100 ° C, stirred for 15 minutes, TLC monitoring to the disappearance of 4-nitrobenzaldehyde, through column chromatography, a white solid was obtained to obtain 2 -(4-nitrophenyl)-[1,3]dithiapentane 0.408 g, yield 89.9%. The reaction is shown in the following formula:
[0034]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com