White spot syndrome virus on-spot detection test paper, its preparation and method of application
A technology for on-site detection and virus, which is applied in the interdisciplinary field of immunology and virology, can solve the problems of fast, on-site detection, time-consuming, high sensitivity and easy to lead to false positives, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Described monoclonal antibody E colloidal gold labeling method is:
[0020] (1) Preparation of colloidal gold: preparation of 18-20nm colloidal gold particles, mixing 100ml of 0.01% chloroauric acid solution with 2.5ml of 0.1% sodium citrate, heating to 100°C to make a colloidal gold solution, the pH value of the solution: Adjust to 8.0-8.4 with 0.2% potassium carbonate, set aside;
[0021] (2) Preparation of gold-labeled monoclonal antibody E:
[0022] Add 1 μl of antibody to 1ml of colloidal gold, mix well, add 100 μl of 10% sodium chloride, observe the color change, if it turns blue, it means that the antibody is insufficient, and then continue to increase the amount of antibody until the color of the colloidal gold does not change. On this basis, increase the amount of this antibody by 50-100%, which is the optimal amount of monoclonal antibody labeled with 1ml of colloidal gold. Colloidal gold is labeled with this appropriate amount of monoclonal antibody, and th...
Embodiment 2
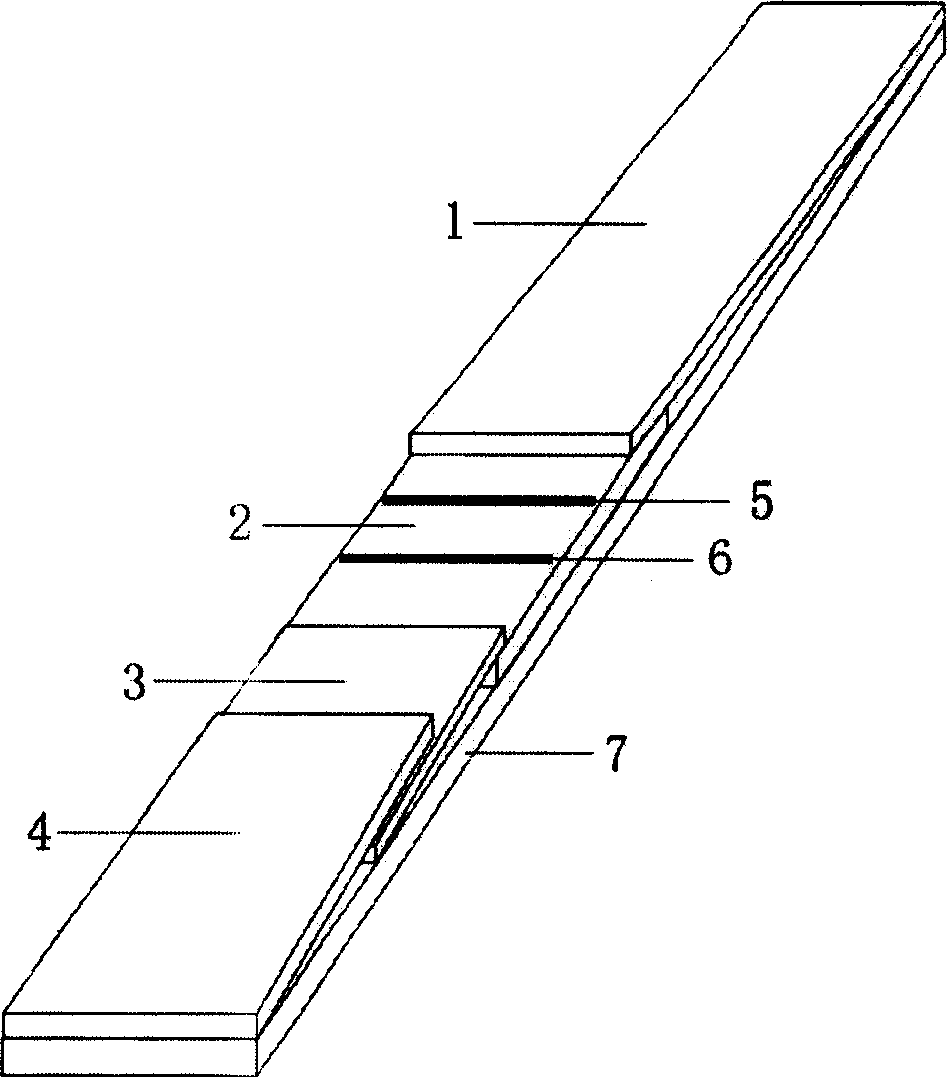
[0024] Preparation of glass fiber layer blocks loaded with gold-labeled monoclonal antibody E: Spray the prepared gold-labeled monoclonal antibody E liquid on the glass fiber membrane until the liquid starts to ooze out, freeze-dry, and store at 4°C for use.
Embodiment 3
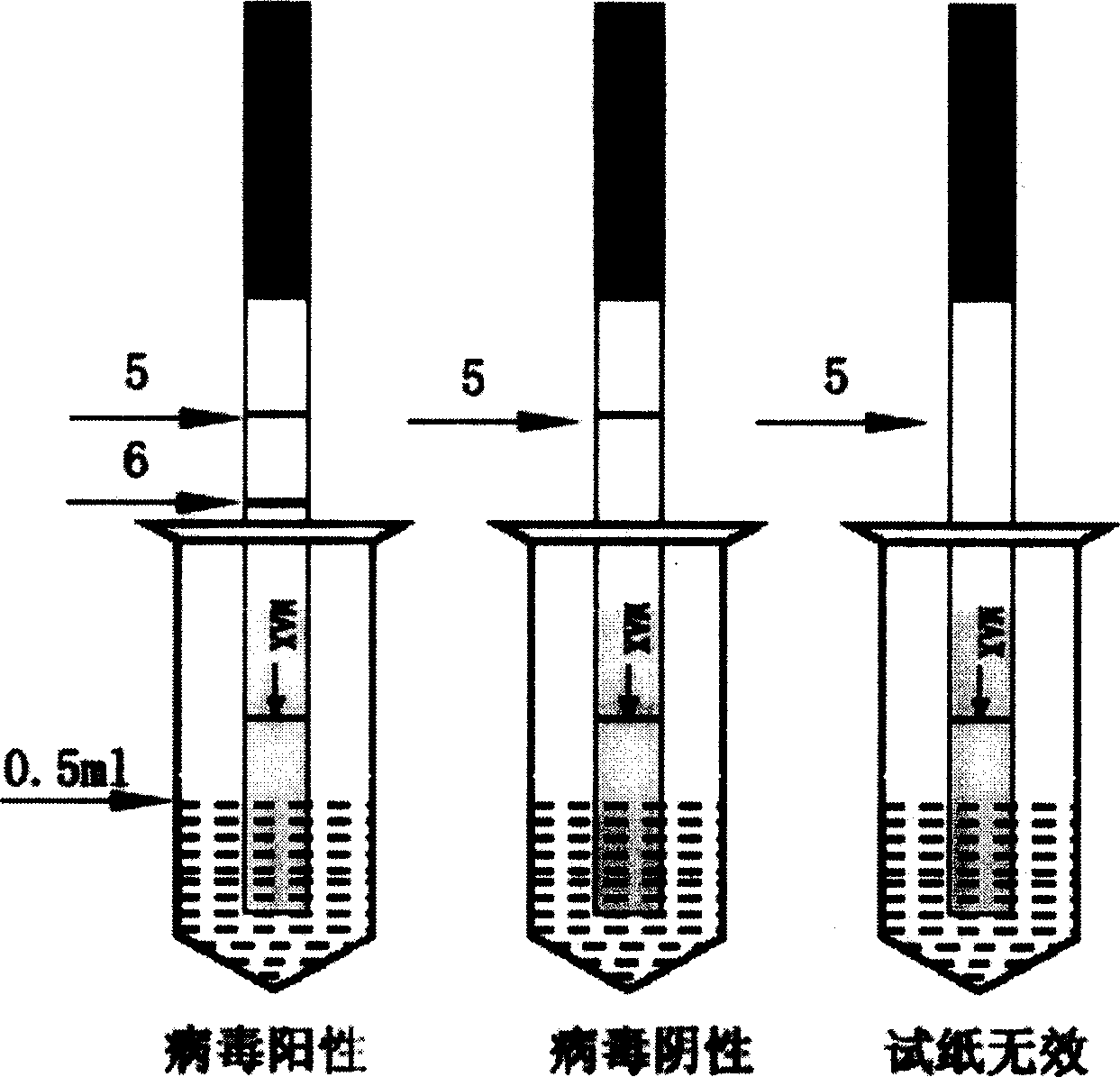
[0026] The preparation method of the detection layer of the white spot virus on-site detection test paper of the present invention is: after the anti-white spot virus monoclonal antibody F purified by the caprylic acid method is freeze-dried, it is prepared into a 4 mg / ml anti-white spot virus monoclonal antibody F liquid, and it is sprayed with a film spraying machine. Spray on the nitrocellulose membrane to form the test line 6; similarly, prepare goat anti-mouse IgG at 300 μg / ml, and spray it on the nitrocellulose membrane with a film spraying machine to form the quality control line 5. The distance between the two lines is 5mm, the quality control line 5 is close to the water-absorbing layer of the hand-held end, and the detection line 6 is close to the water-absorbing layer of the sample loading end. Dry at room temperature, then block with pH7.4, 0.01M PBS containing 10% bovine serum albumin at 37°C for 30min, rinse with PBS and dry in the air.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com