Lanthanum amino acid complex and its preparing process and application
A technology for complexes and amino acids, which is applied in the field of lanthanum amino acid complexes and its preparation, can solve the problems of little work and few literature reports, and achieve the effects of high yield, novel structure design and long life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
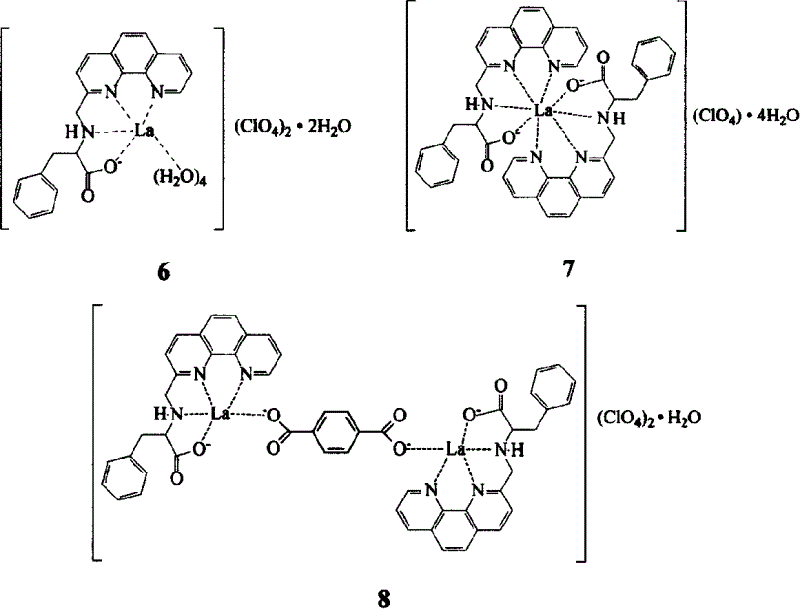
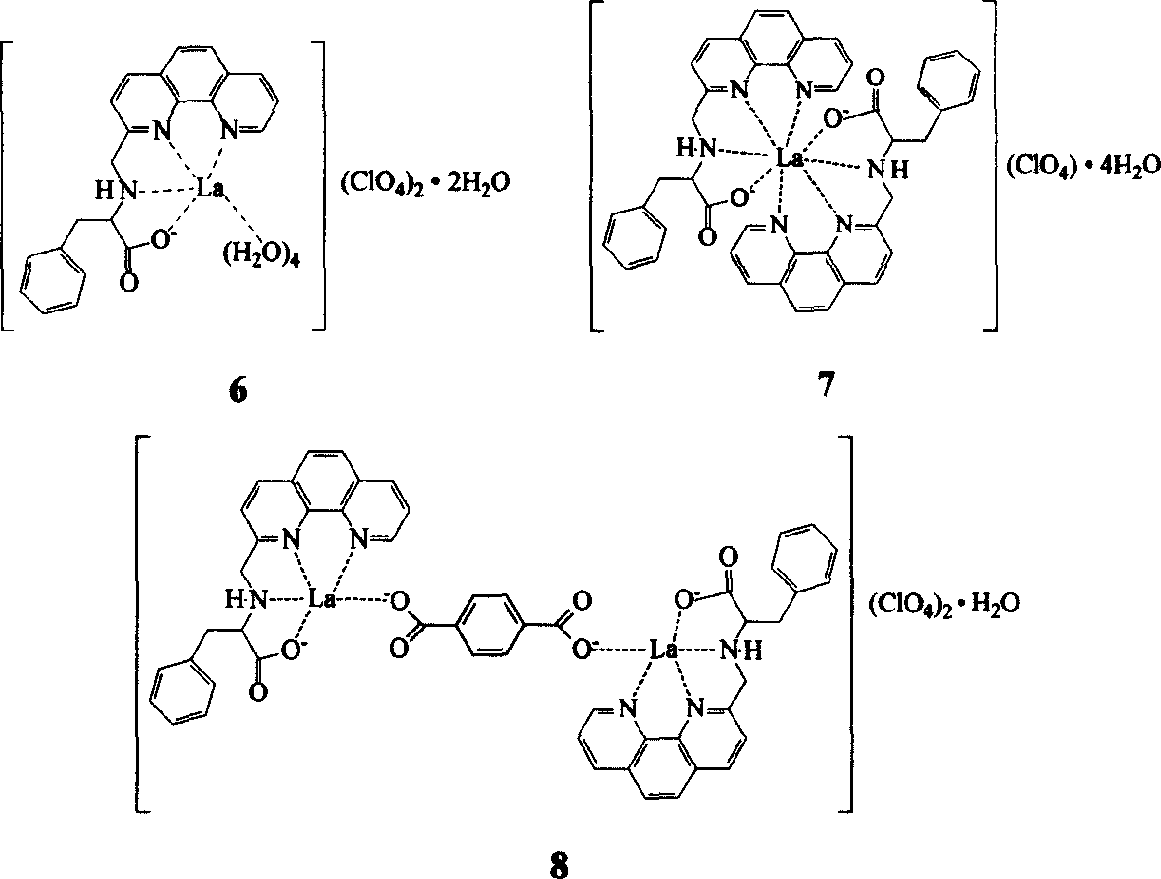
Embodiment 1
[0014] The first step, the synthesis of 8-nitroquinoline (1) (Vogel Arthur. Textbook of practical organic chemistry, 4th ed. London: Longman Group Limited, 1978, 91.)
[0015] 69g (0.5mol) o-nitroaniline, 68.8g (0.3mol) As 2 o 5 Mix well with 138g (1.5mol) glycerol, heat to 100°C, slowly add 120ml concentrated H 2 SO 4 , The speed of addition is such that the reaction temperature is not higher than 120°C. After the addition, the temperature is raised to 130-135°C, and the reaction is continued for 7-8 hours with stirring. After cooling, pour it into 1500ml of water, add 15g of activated carbon, keep stirring at 90°C for 1 hour, filter, and after the filtrate is cooled, neutralize it with 1:1 dilute ammonia water, filter out the crude product, wash with water, and recrystallize in hot water to obtain the product 8- Nitroquinoline 45g, yield 52%, m.p.89-91°C.
[0016] The second step, the synthesis of 8-aminoquinoline (2)
[0017]Mix 50ml of glacial acetic acid and 53ml of ...
Embodiment 2
[0031] The first step, the synthesis of 8-nitroquinoline (1)
[0032]Except that the raw materials used are 69g (0.5mol) o-nitroaniline, 86g (0.375mol) As 2 o 5 With the exception of 184g (2mol) glycerin, other is with embodiment 1.
[0033] The second step, the synthesis of 8-aminoquinoline (2)
[0034] Except adding 9.68g (0.204mol) reduced iron powder, other is the same as embodiment 1.
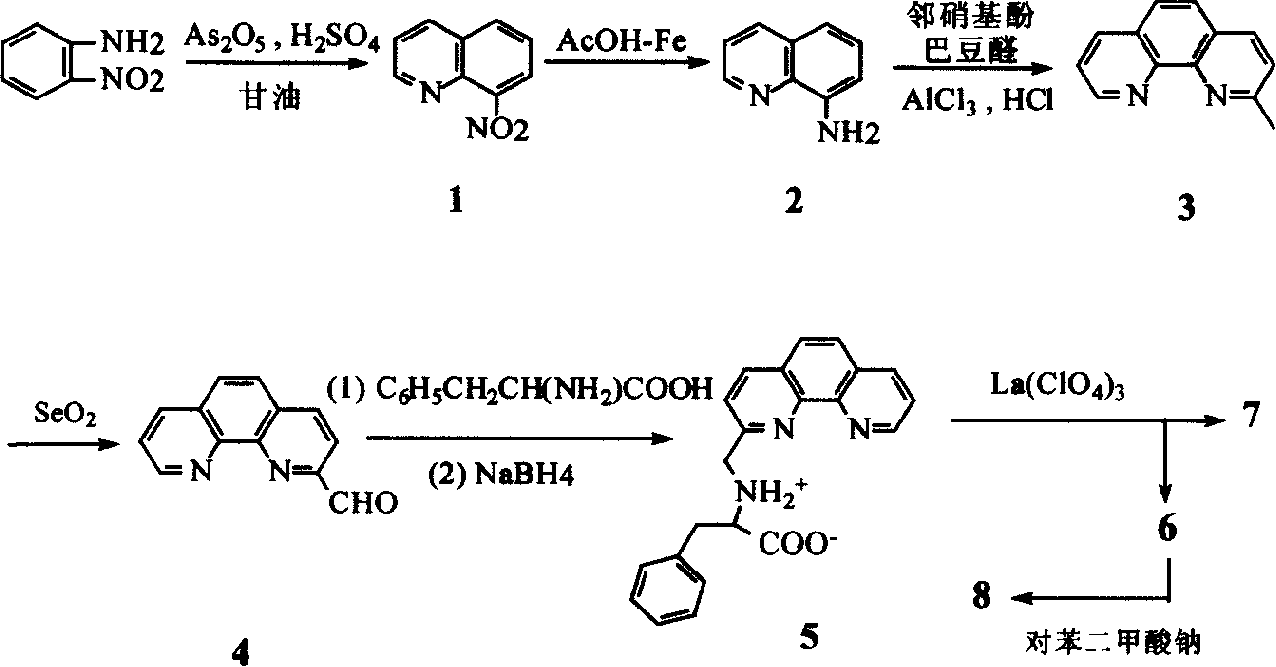
[0035] The third step, the synthesis of 2-methyl-1,10-phenanthroline (3)
[0036] In addition to adding 134g (1mol) of anhydrous AlCl 3 For neutralization, except that 84g (1.2mol) of crotonaldehyde was added dropwise, the others were the same as in Example 1.
[0037] The fourth step, the synthesis of 1,10-phenanthroline-2-carbaldehyde (4)
[0038] In addition to 4.4g (0.04mol) SeO 2 Outside, other is with embodiment 1.
[0039] The fifth step, the synthesis of N-(1,10-phenanthroline-2-methylene)-α-phenylalanine (5)
[0040] In addition to 1.134g (0.03mol) NaBH 4 Outside, other ...
Embodiment 3
[0048] The first step, the synthesis of 8-nitroquinoline (1)
[0049] Except that the raw materials used are 69g (0.5mol) o-nitroaniline, 103.2g (0.45mol) As 2 o 5 And 230g (2.5mol) glycerin, other are with embodiment 1.
[0050] The second step, the synthesis of 8-aminoquinoline (2)
[0051] Except adding 11.4g (0.24mol) reduced iron powder, others are the same as embodiment 1.
[0052] The third step, the synthesis of 2-methyl-1,10-phenanthroline (3)
[0053] In addition to adding 201g (1.5mol) of anhydrous AlCl 3 Neutralization is the same as in Example 1 except that 126g (1.5mol) of crotonaldehyde is added dropwise.
[0054] The fourth step, the synthesis of 1,10-phenanthroline-2-carbaldehyde (4)
[0055] In addition to 5.5g (0.05mol) SeO 2 Outside, other is with embodiment 1.
[0056] The fifth step, the synthesis of N-(1,10-phenanthroline-2-methylene)-α-phenylalanine (5)
[0057] In addition to 1.32g (0.035mol) NaBH 4 Outside, other is with embodiment 1.
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com