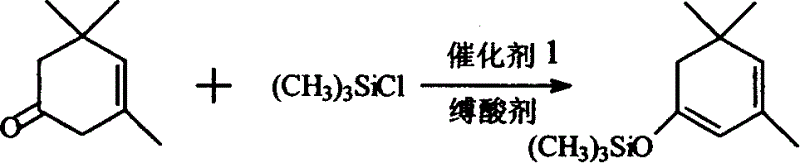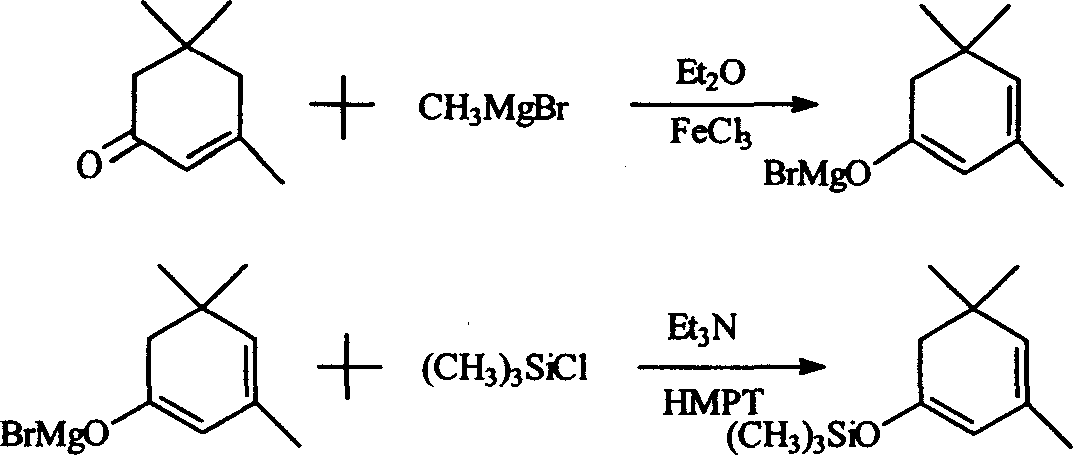Synthetic method for beta-isophorone trimethyl silane enol ether and its use in synthetic large column trienic ketone
A technology of isophorone trimethylsilyl enol ether and isophorone trimethylsilyl ether, which is applied in the synthesis field of beta-isophorone trimethylsilyl enol ether, can solve production difficulties, Problems such as long reaction steps and high toxicity of hexamethylphosphoric triamide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Synthesis of β-isophorone trimethylsilane enol ether: add 400mlDMF and 320g (3.2mol) triethylamine in a 2000ml three-neck flask equipped with stirring, condenser, thermometer and dropping funnel, add under stirring 300g NaI (2mol) and 276g (2mol) of β-isophorone were cooled with ice water, t = about 10°C, 290g (2.7mol) of trimethylchlorosilane was added dropwise, and the temperature was controlled at about 25°C. After the drop was completed, the reaction was stirred at about 25°C for 6 hours, and a large amount of precipitates appeared at the bottom. Filtrate, pour the filtrate into 800g ice water, separate the water layer and extract it with 250ml*2 petroleum ether, combine the petroleum ether liquid and the oil layer, wash with dilute HCl, then wash with brine until neutral, add 10g of anhydrous magnesium sulfate to dry, recover After petroleum ether, distill under reduced pressure, collect the fraction at 68-70°C / 3-4mmHg, and obtain 321g of product, the content is ab...
Embodiment 2
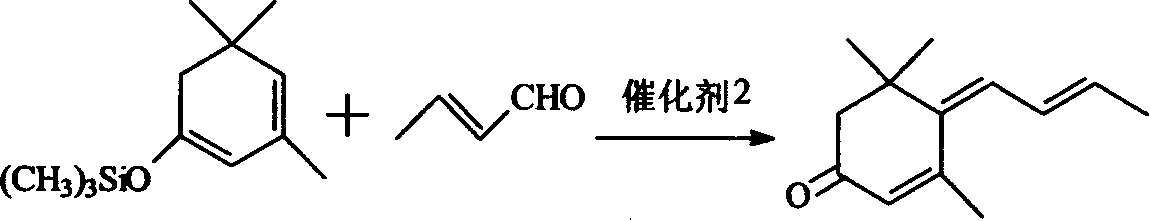
[0022] Application of β-isophorone trimethylsilane enol ether in the synthesis of large column trienone: first synthesize β-isophorone trimethylsilane enol ether with the method and steps of the above-mentioned embodiment one, and then In the 2000ml three-neck flask that is equipped with stirring, condenser, thermometer and dropping funnel, add 321g (90%, 1.37mol) above-mentioned synthetic β-isophorone trimethylsilane enol ether, 500ml anhydrous toluene and 115g (99% 1.64mol) crotonaldehyde, cooled to -25 ° C, began to drop BF 3 .Et 2 O194g (1.37mol), the temperature has risen, and the drop is completed in about 1 hour, the temperature is about -20°C, and the reaction is about -20°C for 8 hours after the drop is completed. Then slowly add 126g Na from the dropping funnel 2 CO 3 Mix the solution with 600g water, stir for 10 minutes, pour it into a 2000ml separatory funnel, separate the water layer, wash the oil layer with water until neutral, recover the toluene, and fractio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com