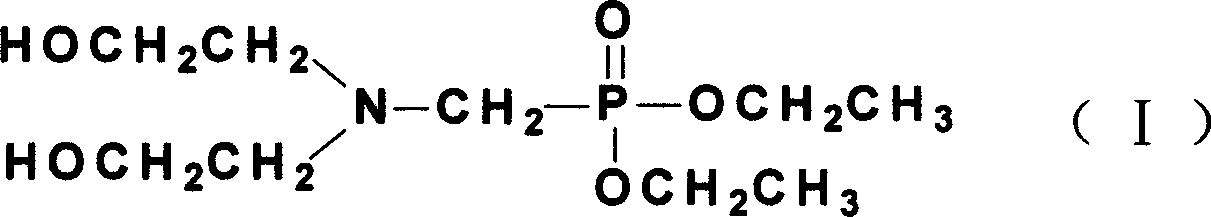
Preparation of N, N-di(2-ethoxyl) aminomethyl diethyl phosphoric acid
A technology of diethyl aminomethylphosphonate and hydroxyethyl, which is applied in the field of preparation of diethyl aminomethylphosphonate, can solve the problems of low yield, difficult separation of by-products, and low content, and achieve a simple preparation method , The effect of improving product quality and improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] (1) Add 165 parts of 37% formaldehyde aqueous solution into the reactor, heat to 30° C., add 206 parts of pre-melted diethanolamine within 30 minutes under stirring. The process of adding diethanolamine is an exothermic reaction, keep the reaction temperature at 30° C., and react for 1 hour.
[0017] (2) Change the reaction device to vacuum distillation. At 50° C., the pressure is less than 0.095 MPa. Water is distilled off under reduced pressure, and then the vacuum distillation is continued for 2 hours at 60° C.
[0018] (3) Change the distillation device back to the reaction device, and lower the temperature to 40°C, add 0.8 parts of anhydrous solid acid catalyst macroporous strong acid cationic resin, add 271 parts of diethyl phosphite within 1 hour under stirring, and then The reaction was incubated at 50°C for 2 hours.
[0019] (4) The reaction material was cooled to room temperature, and the catalyst was recovered by filtration to obtain a yellow transparent liq...
Embodiment 2
[0020] Example 2. (according to USP3,076,010 method)
[0021] (1) Add 24.4g of 37% formaldehyde aqueous solution into the reaction flask, and add 30.9g of diethanolamine at 20°C to 30°C under stirring.
[0022] (2) Add 41.4 g of diethyl phosphite at 20°C to 30°C. Since it is an exothermic reaction, keep the reaction temperature at 35°C under cooling, cool down to 30°C within about 40 minutes, continue stirring at room temperature for a period of time, and then heat to 50°C for 15 minutes.
[0023] (3) Cool the reactant, extract the reactant with 100ml ether, and separate the water layer.
[0024] (4) Concentrate the water layer by distillation under reduced pressure, and then collect the fraction at 50° C. / 1 mmHg by distillation under reduced pressure to obtain the product with a yield of 94.8%. The N,N-bis(2-hydroxyethyl)aminomethylphosphonic acid diethyl ester content in the gas phase analysis product was 88%.
Embodiment 3
[0026] The difference with embodiment 1 is:
[0027] Step (1) Add 40% formaldehyde aqueous solution to the reactor, keep the reaction temperature at 40° C., and react for 1.5 hours.
[0028] Step (2) Change the reaction device to vacuum distillation. At 60° C., the pressure is less than 0.095 MPa. Water is distilled off under reduced pressure, and then continue the vacuum distillation at 80° C. for 2 hours.
[0029] Step (3) Cool down to 50°C, add 1.3 parts of anhydrous solid acid catalyst. Then incubate at 40°C for 3 hours
[0030] A yellow transparent liquid product was obtained with a yield of 99%. The N,N-bis(2-hydroxyethyl)aminomethylphosphonic acid diethyl ester content in the gas phase analysis product was 94%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com