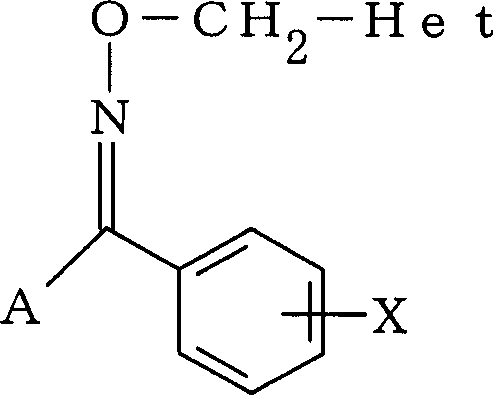
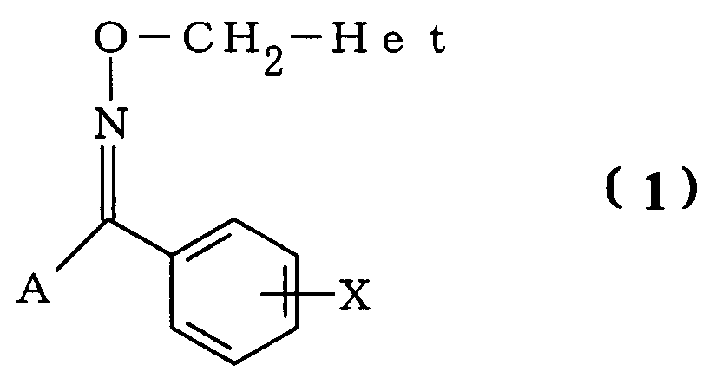Tetrazoyl oxime derivative and agrochemical containing the same as active ingredient
A technology of tetrazolyl oxime and derivatives, applied in the field of pesticides, can solve the problems of no compound usefulness and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0151] The present invention will be described below based on production examples, formulation examples, and test examples, but the present invention is not limited to these.
[0152] First, a production example of a tetrazolyl oxime derivative will be described.
manufacture example 1
[0154] Add 11.1 g (59.1 mmol) of (1-methyltetrazol-5-yl) benzaldehyde and 10.3 g (148 mmol) of hydroxylammonium chloride into 100 ml of pyridine, and stir at 45° C. for 24 hours. After the reaction, the reaction liquid was concentrated under reduced pressure, water and ethyl acetate were added to the obtained residue, and the reaction product was extracted. After washing the organic layer sequentially with dilute hydrochloric acid, water, and aqueous sodium bicarbonate solution, the organic layer was dried over anhydrous magnesium sulfate. By distilling off the solvent from the organic layer, the formula
[0155]
[0156] Represented 12.0 g (100% yield) of (1-methyltetrazol-5-yl)benzoxime.
[0157] 1 H-NMR (CDCl 3 , δ): 4.03(s, 3H), 7.3~7.55(m, 5H), 9.0(brd, 1H).
manufacture example 2
[0159] In Production Example 1, except that 560 mg (2.52 mmol) (1-methyltetrazol-5-yl) 4-chlorobenzaldehyde was used instead of (1-methyltetrazol-5-yl) benzaldehyde, the same as in the production Example 1 is the same, thus obtained the formula
[0160]
[0161] 600 mg of (1-methyltetrazol-5-yl) 4-chlorobenzoxime.
[0162] 1 H-NMR (CDCl 3 , δ): 4.04(s, 3H), 7.36(m, 2H), 7.46(m, 2H), 9.00(brd, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com