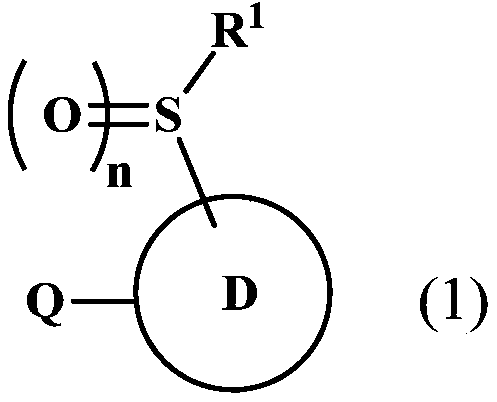
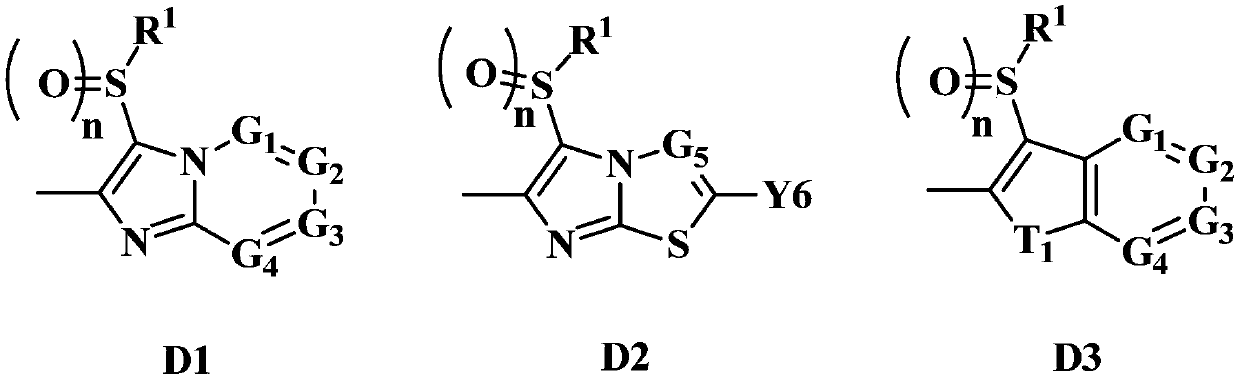
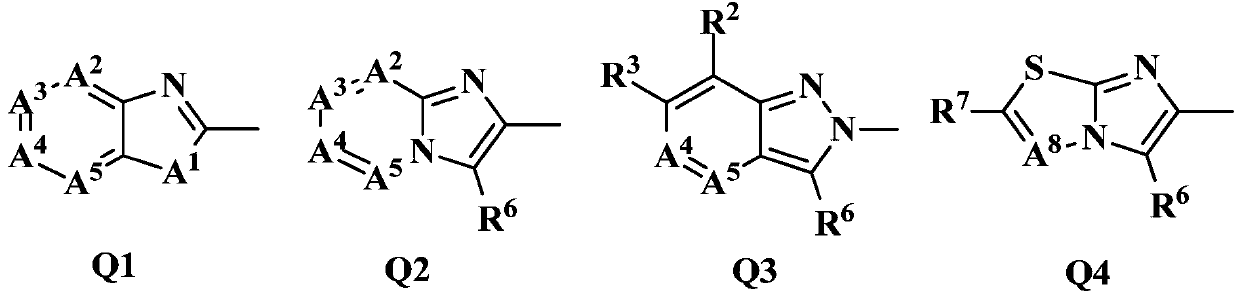
Fused Heterocyclic Compounds and Pest Control Agents
A technology of fused heterocycles and compounds, applied in the field of pest control agents, can solve the problems that fused heterocycles have no disclosure and usefulness is not known
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[1485] The present invention will be further described in detail below by describing the synthesis examples and test examples of the compounds of the present invention as examples, but the present invention is not limited thereto.
[1486] The medium-pressure fractionation liquid chromatography described in the synthesis example and the reference example used the medium-pressure fractionation apparatus of Yamazen Co., Ltd.; YFLC-Wprep (flow rate 18 ml / min, silica gel 40 μm column).
[1487] In addition, the chemical shift value of the proton nuclear magnetic resonance (NMR) in the synthesis example and the reference example uses Me 4 Si (tetramethylsilane) was measured at 300 MHz (model; ECX300 or ECP300, manufactured by JEOL Corporation) as a reference substance.
[1488] The symbols in the proton nuclear magnetic resonance chemical shift values have the following meanings.
[1489] s: singlet, brs: broad singlet, d: doublet, dd: double doublet, t: triplet, q: quartet, m: ...
Synthetic example 1
[1491]Synthesis Example 1: 2-[3-(ethylsulfonyl)-7-(trifluoromethyl)imidazol[1,2-a]pyridin-2-yl]-7-(trifluoromethyl)imidazol[1 , 2-c] the synthesis of pyrimidine (compound No.1-3-001a of the present invention)
[1492] Dissolve 82 mg of 6-(trifluoromethyl)pyrimidin-4-amine in 5 ml of chlorobenzene, and add 2-bromo-1-[3-(ethylsulfonyl)-7-(trifluoromethyl)imidazole at room temperature [1,2-a]pyridin-2-yl]ethanone 200mg. After the addition was complete, the reaction mixture was stirred with heating at reflux for 9 hours. After completion of the reaction, 10 ml of a 1M aqueous sodium hydroxide solution was added to the reaction mixture, followed by extraction with ethyl acetate (10 ml×2). The obtained organic layer was dehydrated and dried in the order of saturated brine, then anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The obtained residue was purified by medium-pressure fractionation liquid chromatography eluted with n-hexane-ethyl aceta...
Synthetic example 2
[1495] Synthesis Example 2: 2-[3-(ethylthio)-7-(trifluoromethyl)imidazo[1,2-a]pyridin-2-yl]-3-methyl-6-(trifluoromethyl )-3H-imidazol[4,5-b]pyridine (compound No.1-1-002b of the present invention) and 2-[3-(ethylsulfonyl)-7-(trifluoromethyl)imidazol[1, Synthesis of 2-a]pyridin-2-yl]-3-methyl-6-(trifluoromethyl)-3H-imidazo[4,5-b]pyridine (Compound No.1-1-002a of the present invention)
[1496] Step 1: 3-(ethylthio)-N-[2-(methylamino)-5-(trifluoromethyl)pyridin-3-yl]-7-(trifluoromethyl)imidazol[1,2 -a] Synthesis of pyridine-2-carboxamide
[1497] Will N 2 -Methyl-5-(trifluoromethyl)pyridine-2,3-diamine 856mg was dissolved in 20ml of pyridine, and 3-(ethylthio)-7-(trifluoromethyl)imidazole[1, 2-a] 1.00 g of pyridine-2-carboxylic acid, 1.32 g of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride and 42 mg of 4-(dimethylamino)pyridine. After the addition was complete, the reaction mixture was stirred at room temperature for 6 hours. After completion of the reaction, t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com