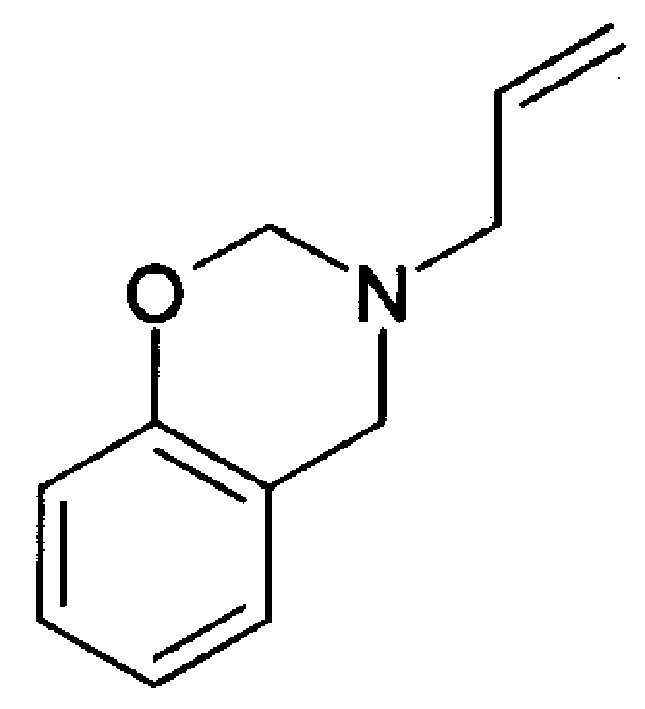
Benzoxazine intermediate containing N-allyl and composition and preparation thereof
A technology of benzoxazine and intermediates, applied in the direction of organic chemistry and the like, can solve the problems of difficult to obtain, high ring-opening temperature, difficult ring-opening polymerization, etc., and achieves improved glass transition, improved heat resistance, and improved cross-linking. The effect of density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0045] Then it is cured. The curing temperature and time are: 2 hours at 110°C, 2 hours at 180°C, 2 hours at 200°C, 2 hours at 220°C and 2 hours at 240°C to obtain the curing After thermal analysis, its glass transition temperature (Tg) reached 284°C (determined by DMA method). Example 2 Preparation of 2,2-bis(3,4-dihydro-3-allyl-1,3-benzoxazine) propane, that is, bisphenol A type N-allylbenzoxazine, by melting method (The structural formula is as follows, referred to as ABPA)
[0046] Add (15ml, 0.2mol) allylamine to bisphenol A (22.83g, 0.1mol) at room temperature, stir at low temperature (2℃) until the system is a transparent and homogeneous mixture; add in batches (12.00g, 0.4mol) ) Paraformaldehyde, continue to stir for 10 min (according to the molar ratio of phenolic hydroxyl, amine and aldehyde functional groups, it is 1:1:2). The temperature was gradually increased to reflux, and the reaction was stopped after 2.5 hours of reaction. Cool to obtain a milky white solid; the...
Embodiment 3
Embodiment 4 Synthetic preparation 2,2-(3,4- 2 -3--1,3-)AN-(,ABPA) approach and Embodiment 2,A、、1∶2.4∶4.8(、、1∶1.2∶2.4),2,2-(3,4- 2 -3--1,3-)2,2-(3,4- 2 -3--1,3-),98%;H-NMR,98%。 Embodiment 5
[0048]The method is the same as in Example 2, except that the molar ratio of bisphenol, allylamine A, and paraformaldehyde is 1:2.2:4.4 (according to the molar ratio of phenolic hydroxyl group, amine group, and aldehyde group, it is 1:1.1: 2.2), 2,2-bis(3,4-dihydro-3-allyl-1,3-benzoxazine) propane is prepared as light yellow crystals, namely 2,2-bis(3,4-di Hydroxy-3-allyl-1,3-benzoxazine) propane, the yield is 99%; through H-NMR measurement, the ring formation rate is 100%. Example 4 Preparation of 2,2-bis(3,4-dihydro-3-allyl-1,3-benzoxazine)propane, namely bisphenol A type N-allylbenzoxine, by a melt synthesis method The method of oxazine (see below for structural formula, ABPA for short) is the same as in Example 2, except that the molar ratio of bisphenol A, allylamine, and paraformaldehyde is 1:2.4:4.8 (according to phenolic hydroxyl group, amino group, and aldehyde group). The molar ratio is 1:1.2:2.4), and 2,2-bis(3,4-dihydro-3-allyl-1,3-benzoxazine) propane is obtained as l...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com