Preparation of nitrile compounds
A compound and organic compound technology, which is applied in the field of preparation of polynitrile compounds, can solve the problems of single-flow yield drop, low reaction rate, and reduced productivity of nitrile compounds, and achieve the goals of suppressing side reactions, reducing losses, and high yields Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
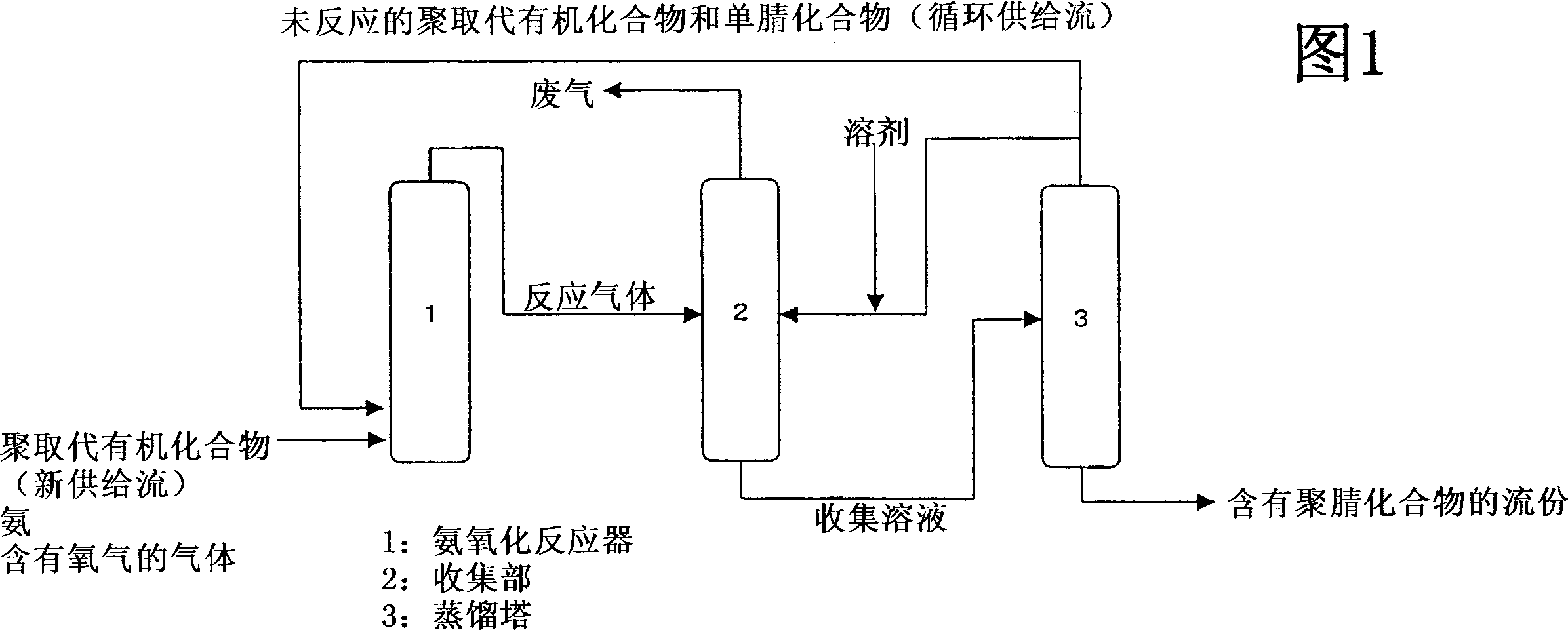
[0040] The ammoxidation reaction of m-xylene was carried out using the ammoxidation reactor shown in FIG. 1 . 2.3 tons of the above-prepared mobile catalyst were filled in an ammoxidation reactor, and air, m-xylene (MX), and ammonia gas were preheated at 180°C and supplied to the reactor. Supply rate is MX 266kg / hr, MTN 40.0kg / hr, ammonia 412kg / hr, air 1570Nm 3 / hr. The reaction is carried out at a reaction pressure of 0.08Mpa to obtain isophthalonitrile (IPN). The reaction temperature was set at 427°C. The freshly supplied MX (new supply flow) was substantially the same as the comparative example, 241.8 kg / hr, the circulating supply flow MX was 24.2 kg / hr, and the MTN was 40.0 kg / hr. The molar ratio of the flow rate of the mononitrile compound at the reactor outlet to the total flow rate of the poly-substituted organic compound and the mononitrile compound supplied to the reactor was 12.0 mol%.
[0041] The reaction gas was analyzed, containing MX 24.2kg / hr, IPN 257.2kg / h...
Embodiment 2
[0044] The ammoxidation reaction of m-xylene was carried out using the ammoxidation reactor shown in FIG. 1 . 2.3 tons of the above-prepared mobile catalyst were filled in an ammoxidation reactor, and air, m-xylene (MX), and ammonia gas were preheated at 180°C and supplied to the reactor. Supply rate is MX245.6kg / hr, MTN 10.7kg / hr, ammonia 350kg / hr, air 1320Nm 3 / hr. The reaction is carried out at a reaction pressure of 0.08Mpa to obtain isophthalonitrile (IPN). The reaction temperature was set at 427°C. The freshly supplied MX (new supply flow) was 242 kg / hr as in the comparative example, the circulating supply flow MX was 3.6 kg / hr, and the MTN was 10.7 kg / hr. The molar ratio of the flow rate of the mononitrile compound at the reactor outlet to the total flow rate of the MX poly-substituted organic compound and the mononitrile compound supplied to the reactor was 3.8 mol%.
[0045] The reaction gas was analyzed, containing MX 3.6kg / hr, IPN 247kg / hr, MTN 10.7kg / hr.
[00...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com