Humanized anti-ErbB2 antibodies and treatment with anti-ErbB2 antibodies
A humanized antibody, antibody technology, applied in the direction of antibodies, anti-tumor drugs, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
[0260] Controlled release formulations can also be prepared. Suitable examples of controlled-release preparations include semi-permeable matrices of solid hydrophobic polymers containing antibodies, which matrices are shaped articles such as films or microcapsules. Examples of controlled-release preparations include polyesters, hydrogels (such as poly(2-hydroxyethyl-methacrylate) or poly(vinyl alcohol), polylactide (US Patent 3,773,919), L-glutamic acid and gamma ethyl -L-glutamate copolymer, non-degradable ethylene ethyl acetate, degradable lactic acid-glycolic acid copolymer such as LUPRONDEPOT TM (Injectable microspheres composed of lactic acid-glycolic acid copolymer and leuprolide acetate), and poly-D-(-)-3-hydroxybutyric acid.
[0261] The preparation for in vivo administration must be sterile. This can be easily achieved by filtration through a sterile filter.
[0262] V. Treatment with anti-ErbB2 antibodies
[0263] According to the present invention, anti-ErbB2 antibodies...
Embodiment 1
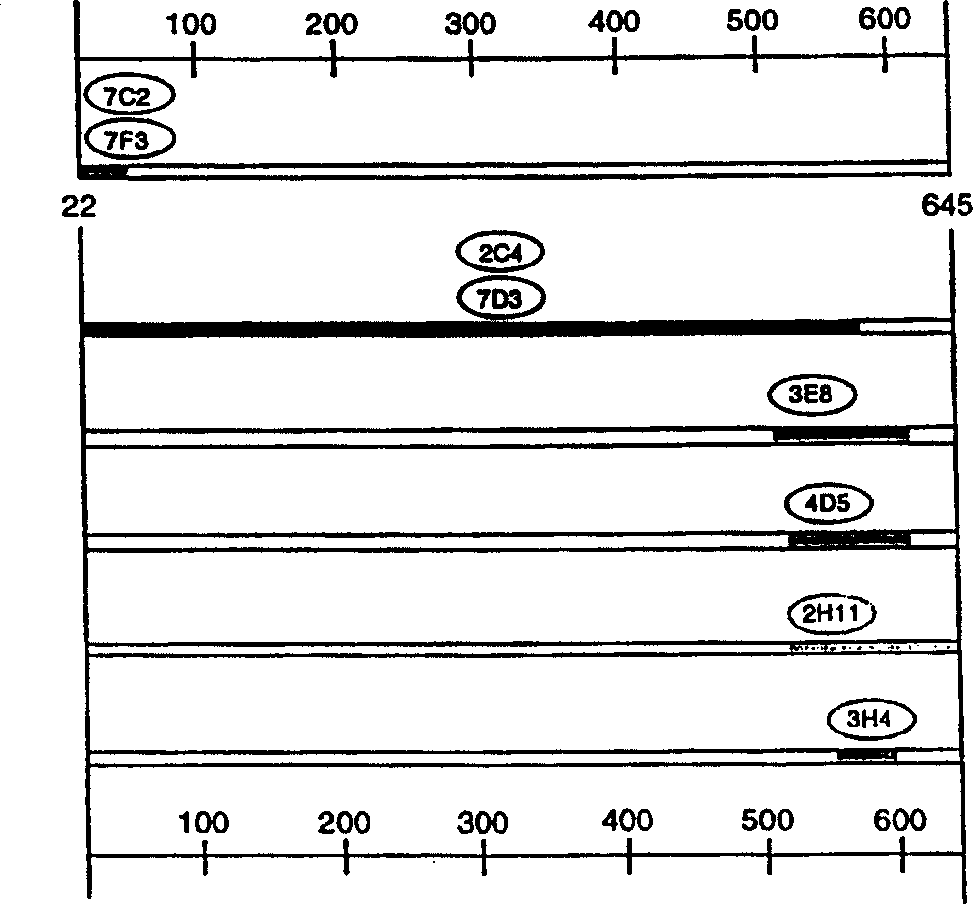
[0323] Preparation and identification of monoclonal antibody 2C4
[0324]According to the method described in Fendly et al., Cancer Research 50: 1550-1558 (1990), murine monoclonal antibodies 2C4, 7F3, and 4D5 that specifically bind to the extracellular domain of ErbB2 were prepared. In short, NIH 3T3 / HER2-3400 cells (about expression 1×10 5 ErbB2 molecule / cell) and used it to immunize BALB / c mice. In 0, 2, 5 and 7 weeks, mice were injected intraperitoneally with 10 7 Cells (in 0.5ml PBS). Give a specific antiserum (which can be combined with 32 P-labeled ErbB2 immunoprecipitation) mice were intraperitoneally injected with wheat germ agglutinin-Sepharose (WGA) purified ErbB2 membrane extract. Then 0.1ml of ErbB2 preparation was injected intravenously, and the spleen cells were fused with the mouse myeloma cell line X63-Ag8.653.
[0325] The ErbB2 binding of the supernatant of hybridoma cells was screened by ELISA and radioimmunoprecipitation.
[0326] A competitive binding assay (...
Embodiment 2
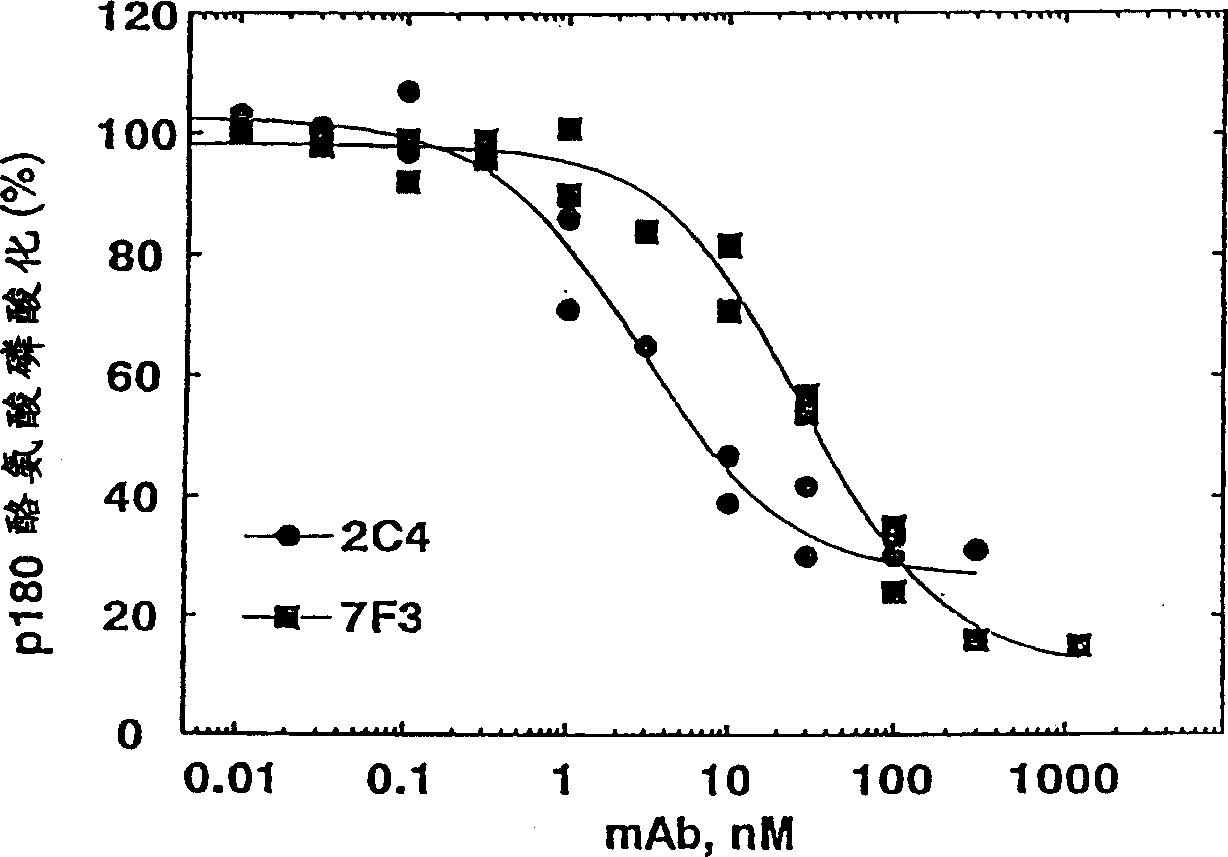
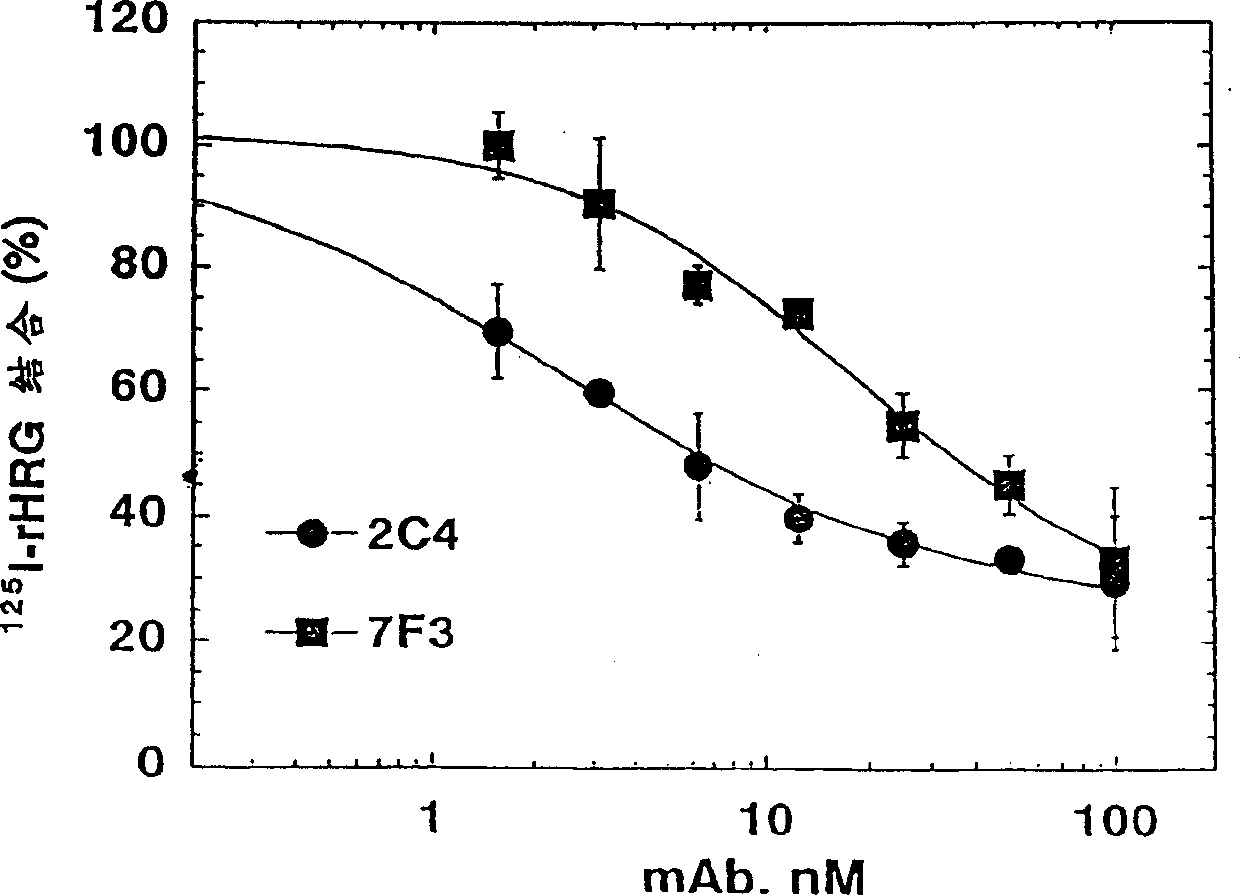
[0336] Monoclonal antibody 2C4 blocks the HRG-dependent binding of ErbB2 to ErbB3
[0337] Co-immunoprecipitation was used to detect the ability of ErbB3 to bind ErbB2. 1.0×10 6 MCF7 or SK-BR-3 cells were seeded in a 6-well tissue culture plate and allowed to adhere overnight. The culture medium was 50:50 DMEM / Ham's F12 containing 10% fetal bovine serum (FBS) and 10mM HEPES (pH 7.2) Base (growth medium). Before the start of the experiment, the cells were starved and cultured in serum-free growth medium for 2 hours.
[0338]Simply wash the cells with PBS, and then use 100 nM of the indicated antibody (diluted in 0.2% w / v bovine serum albumin (BSA) RPMI medium (pH 7.2) (binding buffer) containing 10 mM HEPES) or just The cells were incubated with binding buffer (control). After 1 hour at room temperature, HRG was added to half of the wells to a final concentration of 5nM(+). Add a similar volume of binding buffer (-) to the other wells. Continue to incubate for approximately 10 minu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com