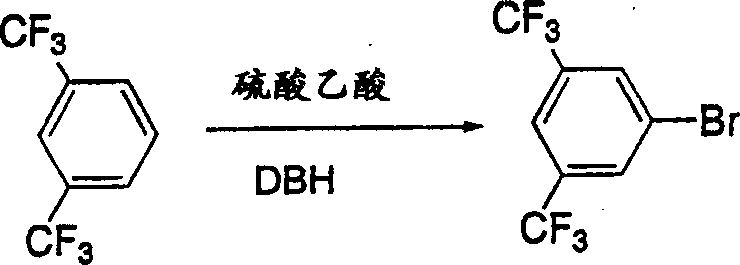
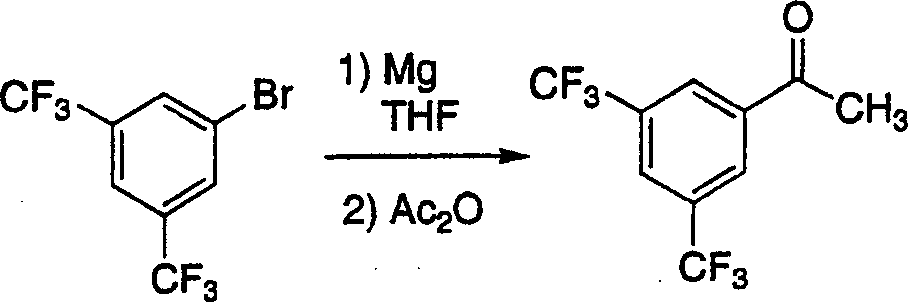
Process for the synthesis (R)-1-(3.5-bis (trifluoromethyl)-phenyl) ethan-1-ol by asymmetric transfer hydrogenation
A technology of trifluoromethyl and phenyl, applied in the field of synthesizing (R)-1-(3,5-bis(trifluoromethyl)-phenyl)ethan-1-ol through asymmetric transfer hydrogenation, capable of Solve problems such as instability and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 3
[0073] Example 3 (R)-1-(3,5-bis(trifluoromethyl)-phenyl)ethan-1-ol 1-(3,5-bis(trifluoromethyl)-phenyl) 256.15 3.9 1Kg ethyl-1-one (Cp*RhCl 2 ) 2 618.08 0.01 6g (Cp*=pentamethylcyclopentadienyl)(S,R)-cis-aminoindanol 149.20 0.02 3.0gNaOH 5N(H 2 O) 0.05 9mlIPA 7LHCl 1N(H 2 O) 7L heptane 7L1,4-diazabicyclo[2.2.2]octane 112.18 2.2 240g (DABCO)
[0074] At room temperature, rhodium salt and ligand are added in the IPA and aged for 0.5 hour. During the aging, the solution usually turns into a transparent orange-red color. After adding the ketone, the alkali is added, and the aging reaction is completed until the reaction is observed by HPLC ( ~3 hours). The reaction was then quenched with 1N hydrochloric acid and extracted with heptane (2 x 3.5 L) and washed with 5 L of brine. DABCO was added and the solution was concentrated to a volume of ~4ml / g alcohol. At this point the KF is less than 200 and less than 5% IPA remains. If these crit...
Embodiment 4
[0075] Example 4 (R)-1-(3,5-bis(trifluoromethyl)-phenyl)ethan-1-ol 1-(3,5-bis(trifluoromethyl)-phenyl)ethane 256.15 3.9 1Kg-1-ketone (Cp*RhCl 2 ) 2 618.08 0.01 6g (Cp*=pentamethylcyclopentadienyl)(R,R)-tosylcyclohexanediamine 268.38 0.02 5.2gNaOH 5N(H 2 O) 0.05 9mlIPA 7LHCl 1N(H 2 O) 7L heptane 7L1,4-diazabicyclo[2.2.2]octane 112.18 2.2 240g (DABCO)
[0076]At room temperature, rhodium salt and ligand are added in the IPA and aged for 0.5 hour. During the aging, the solution usually turns into a transparent orange-red color. After adding the ketone, the alkali is added, and the aging reaction is completed until the reaction is observed by HPLC ( ~3 hours). The reaction was then quenched with 1N hydrochloric acid and extracted with heptane (2 x 3.5 L) and washed with 5 L of brine. DABCO was added and the solution was concentrated to a volume of ~4ml / g alcohol. At this point the KF is less than 200 and less than 5% IPA re...
Embodiment 5
[0077] Example 5 (R)-1-(3,5-bis(trifluoromethyl)-phenyl)ethan-1-ol Material Mw Mol dosage 1-(3,5-bis(trifluoromethyl)-phenyl)ethan-1- 256.15 11.7 3Kg ketone [RuCl 2 (paramene)] 2 612.40 0.03 18.4g (Cym=p-Cymene (4-isopropyltoluene) (S, R)-cis-aminoindanol 149.20 0.06 9.0gNaOH 5N(H 2 O) 0.14 28mlIPA 21LHCl 1N(H 2 O) 21L heptane 21L1,4-diazabicyclo[2.2.2]octane 112.18 ~6.6 ~740g (DABCO)
[0078] Ruthenium salt [RuCl 2 (paramene)] 2 and (S, R)-cis-aminoindanol were added to IPA and aged for 0.5 hours. During the aging, the solution usually turned into a transparent orange, and 1-(3,5-bis(trifluoro Methyl)-phenyl)ethan-1-one and degas the reaction under vacuum. Base was then added and the reaction was aged until >98% complete by HPLC (4-6 hours). The reaction was then quenched by pouring into 1N hydrochloric acid and extracted with heptane (2 x 10.5 L) and washed with 15 L of brine. 1,4-Diazabicyclo[2.2.2]octane (DABCO) was add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com