Methods for detecting membrane derived caspase activity and modulators thereof
A technology of activity and source, applied in the direction of biochemical equipment and methods, measuring devices, microbial determination/testing, etc., can solve the problems of lack of specificity, effectiveness and practicability, method limitations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Cell Lines and Cell Culture
[0096]Stable transfections containing Bcl-2 cDNA (697-Bcl-2 cells) or control neomycin resistance gene (697-neo cells) (Miyashita and Reed, 1993) (obtained from Dr. John Reed , Burnham Institute) retroviral expression constructs of 697 human lymphoblastoid cells. These cells were treated with 10% fetal bovine serum ((FBS)Hyclone, Logan, UT), 0.2 mg / ml G-418 (Gibco, Gaithersburg, MD) and 0.1 mg / ml penicillin / streptomycin (Irvine Scientific, Growth in mid-log phase was maintained in RPMI 1640 medium (Irvine Scientific) in Santa Aha, Ca). Murine dopaminergic MN9D cells (obtained from Dr. A. Heller, University of Chicago) were cultured in DMEM medium (Irvine Scientific) supplemented with 10% FBS, 2 mM glutamine and 0.1 mg / ml penicillin / streptomycin. Pregnant E15 mouse cerebral cortex cells were prepared in Hank's buffered saline solution (Irvine Scientific) containing 15 mM HEPES. The tissue was briefly dissociated with 0.1% trypsin, then th...
Embodiment 2
[0098] subcellular fractionation
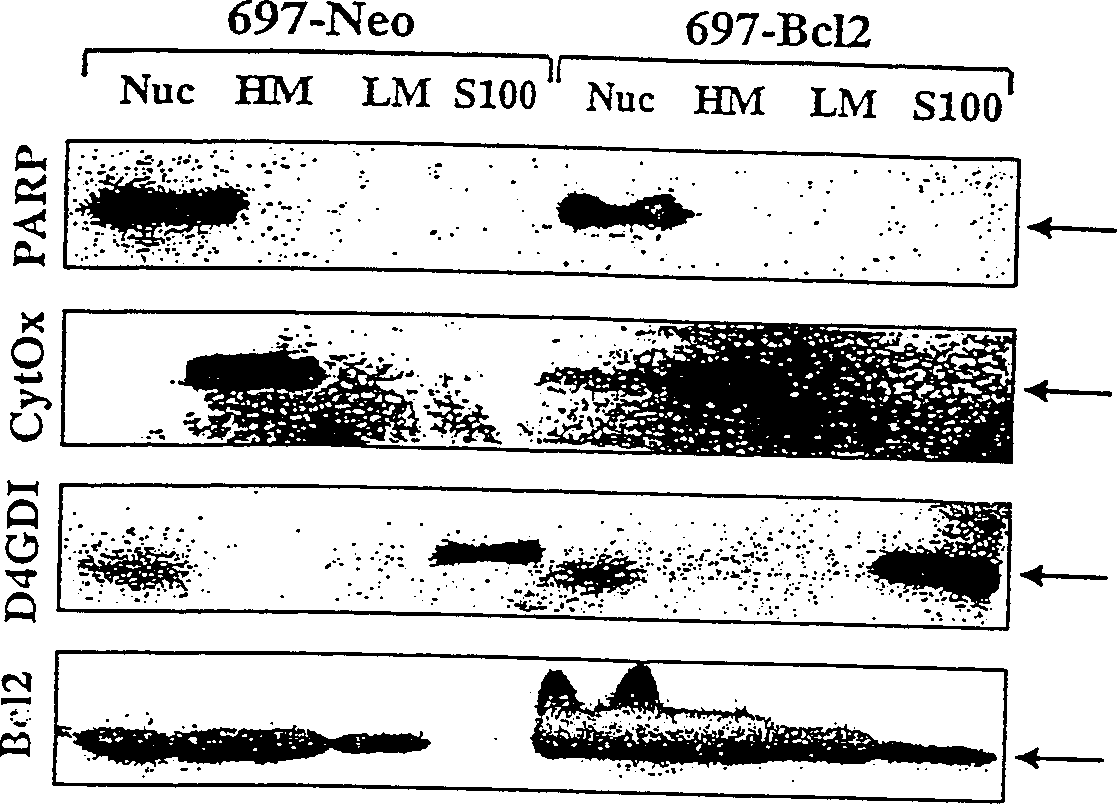
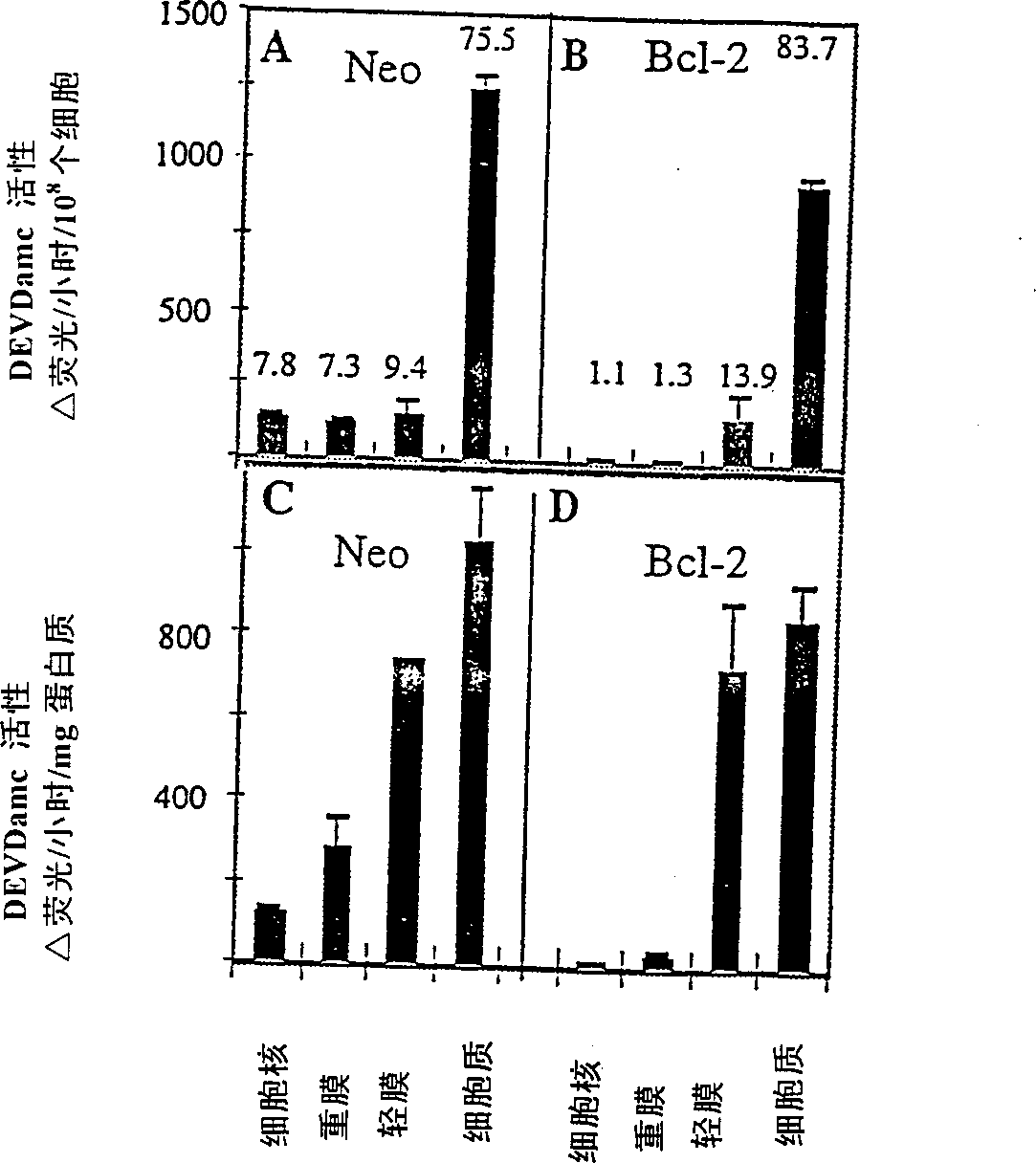
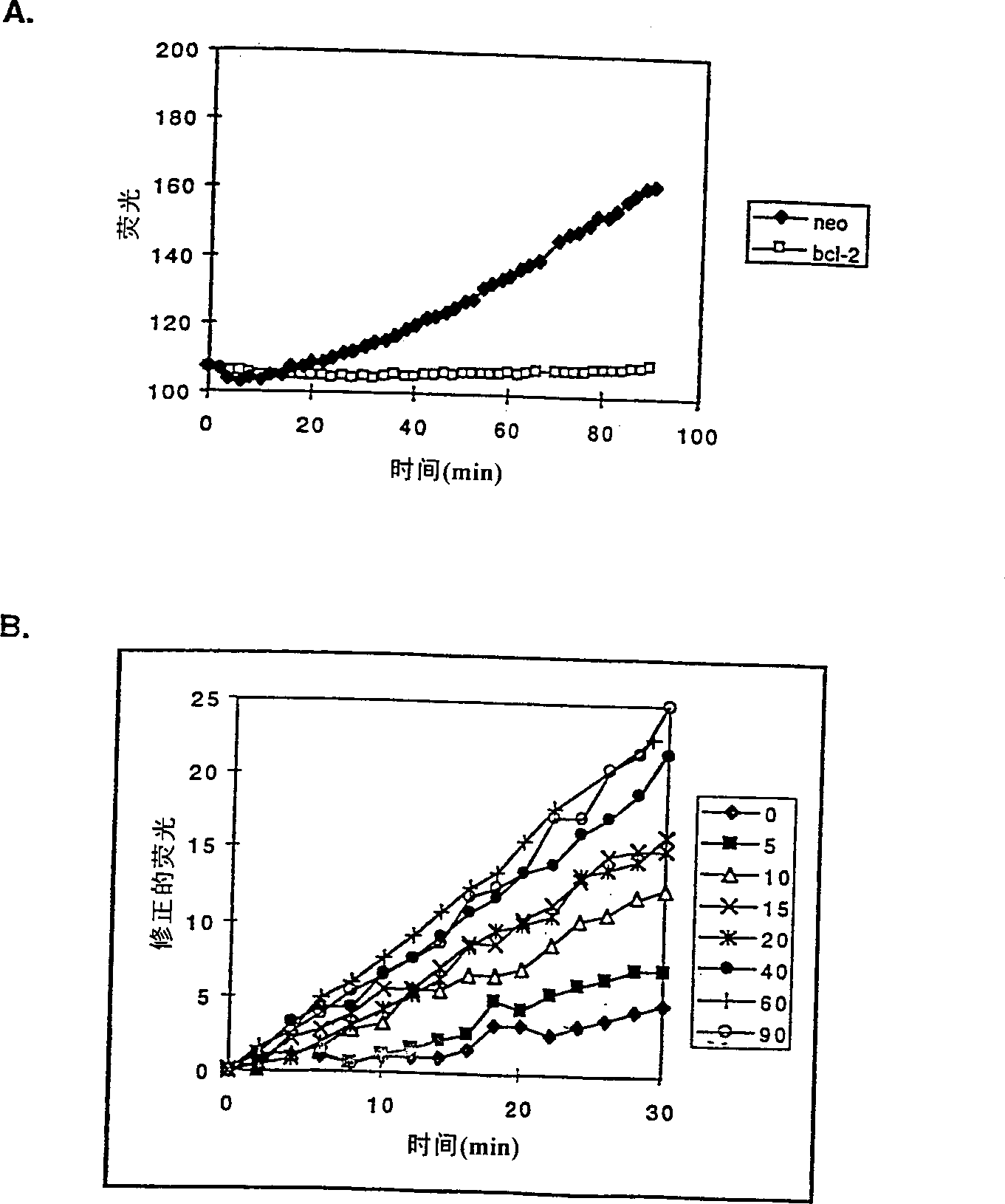
[0099] will contain ≈10 9 Frozen cell pellets of two cells were thawed and resuspended in supplemented with 1 mM PMSF, 1 μg / ml leupeptin, 1 μg / ml pepstatin A, 5 μg / ml aprotinin, 0.1 mM EDTA, 0.1 mM EGTA and 5mM DTT (Sigma) in cold hypotonic buffer (10mM Na-HEPES, 5mMMgCl 2 , 42mM KCl, pH 7.4), the density is ≈1.5×10 8 cells / ml. The samples were incubated on ice for 30 minutes during which time the cells were lysed with 30-40 strokes of a Dounce homogenizer. The samples were centrifuged twice at 500 xg for 10 minutes at 4°C to isolate cell nuclei. The nuclear pellet was then washed twice in the same buffer supplemented with 1.6M sucrose to yield the nuclear fraction. The supernatant from the above centrifugation step was then centrifuged at 14,000×g, 4°C for 30 minutes to pellet the heavy membrane. The heavy membrane was washed 3 times with 1.5 ml of cold hypotonic buffer containing protease inhibitors and DTT. Washed membranes were res...
Embodiment 3
[0101] western blot
[0102] Subcellular fractions (50 μg protein per lane) were separated by SDS-PAGE on 12% or 16% gels (Novex, La Jolla, CA) and then transferred to Immobilon PVDF membranes (Millipore, Bedford, MA) . Membranes were blocked in PBS / 0.1% Tween 20 (PBST) + 0.4% casein (I-block, Tropix, Bedford, MA). Incubate blots for 1 hr in 1 μg / ml primary antibody diluted in PBST / casein. After 3 washes in PBST, blots were incubated for 1 hour in alkaline phosphatase-conjugated goat anti-rabbit IgG or goat anti-mouse IgG (Tropix) diluted 1:15,000 in PBST / casein. The blot was then washed twice with PBST in assay buffer (10 mM diethanolamine, pH 10.0, 1 mM MgCl 2 ), then incubated with 250 μM chemiluminescence substrate CSPD (Tropix) in assay buffer and exposed to Biomax film (Kodak, Rochester, NY) overnight.
[0103] In some cases, after incubation with the secondary antibody, 10 mM Tris, pH 9.5, 1 mM MgCl 2 Wash blot. The blot was then incubated for 30 minutes in 1.25 μ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com