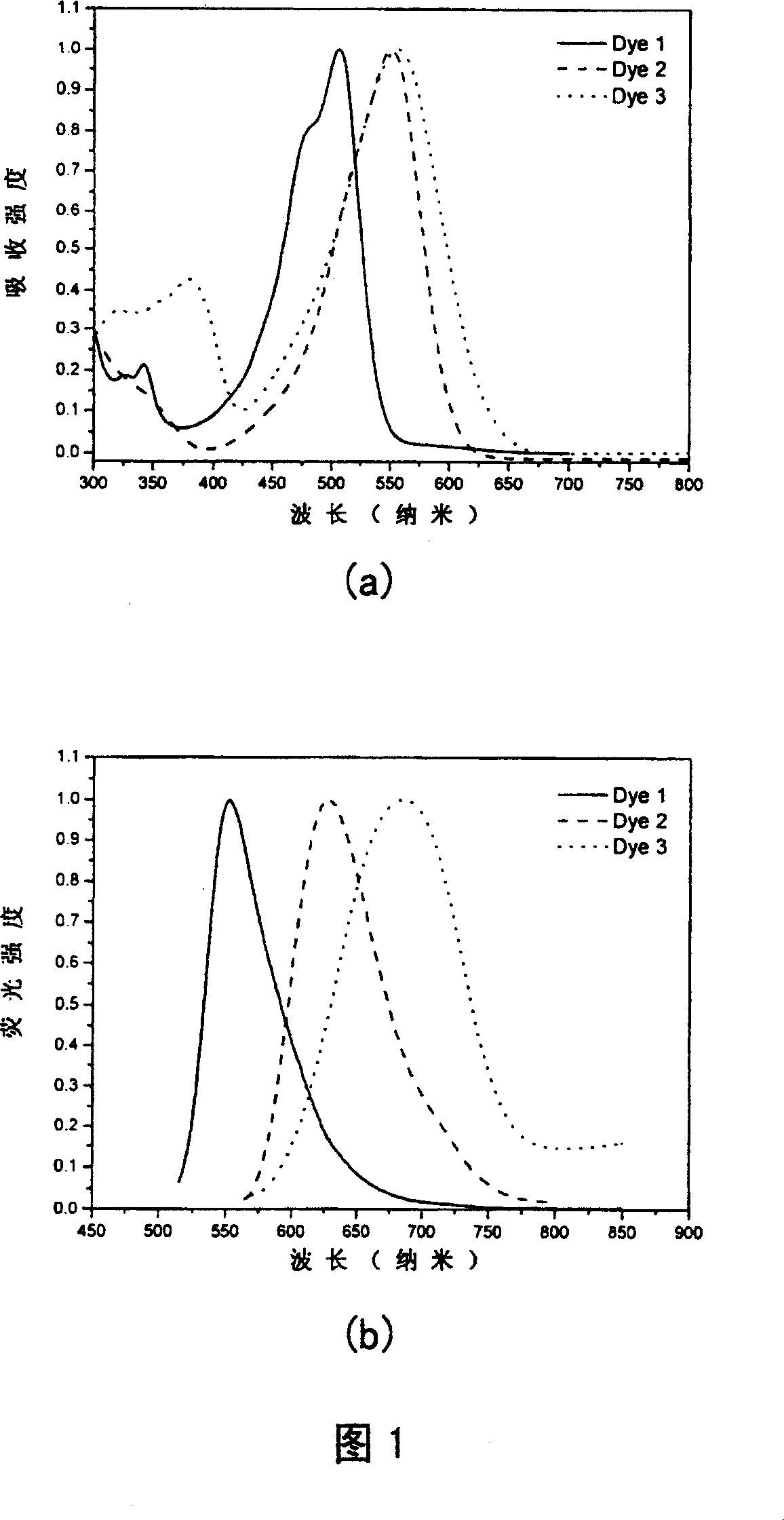
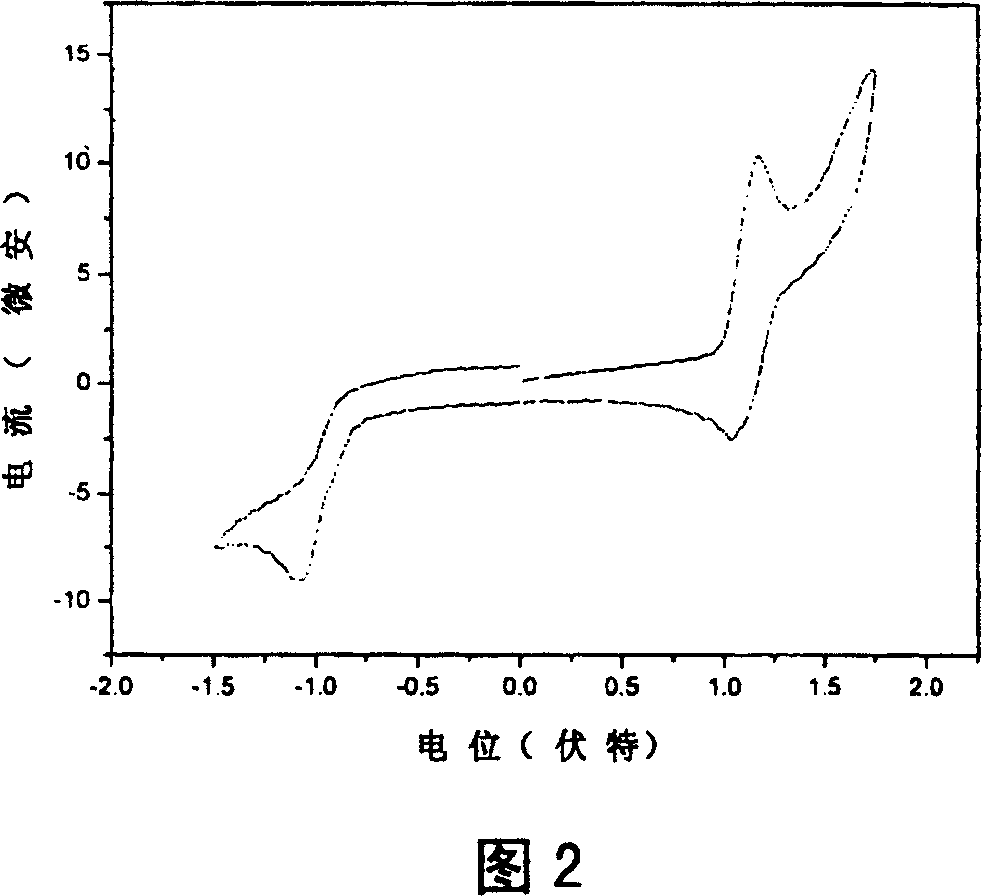
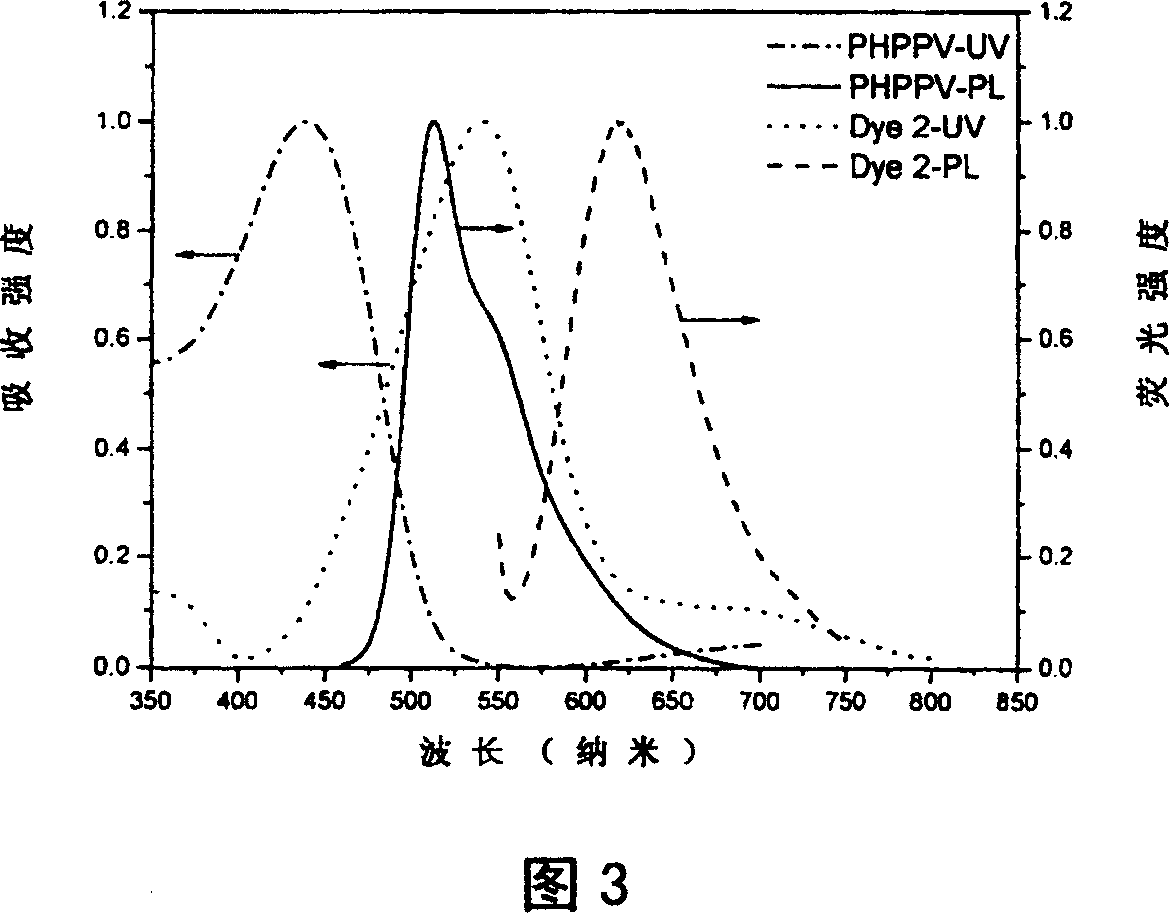
Intermolecular charge transfer type fluorescent dyes and use thereof
A technology for fluorescent dyes and charge transfer, which is applied in the field of intramolecular charge transfer fluorescent dyes, which can solve the problems of lack of materials and achieve the effects of good purity, good solubility and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment (1
[0058] Embodiment (1): N, N'-di-[9-(2'-ethyl-hexyl)-carbazole-vinyl]-succinonitrile (Dye 1)
[0059] In a 100ml three-necked flask, 3-formyl-9-(2'-ethyl-hexyl)-carbazole (42mmol) and diaminosuccinonitrile (20mmol) were placed in about 40ml of glacial acetic acid, and a few Drop acetic anhydride as a catalyst, heat and reflux at 120°C for 5 hours under a nitrogen atmosphere; after the reaction stops, cool to room temperature, pour the mixture into 150ml of water, leave it for 4 hours, and extract the aqueous phase with dichloromethane (60ml×2) ; The organic phases were combined, washed with water, and dried over anhydrous magnesium sulfate. The organic solvent was removed by vacuum rotary evaporation, and the crude product was purified by silica gel column chromatography (eluent-dichloromethane / petroleum ether 1:1 v / v) to obtain a dark red solid with metallic luster (yield 72%).
[0060] Mass Spectrum (MALDI-TOF-MS): m / z calculated 686.4, measured 687.6 (M + );
[0061] H NM...
Embodiment (2
[0063] Embodiment (2): N, N'-two-(triphenylamine)-vinyl-succinonitrile (Dye 2)
[0064] In a 100ml three-necked flask, put aldehyde triphenylamine (42mmol) and diaminosuccinonitrile (20mmol) in about 40ml of glacial acetic acid, add a few drops of acetic anhydride as a catalyst, and heat to reflux at 120°C under a nitrogen atmosphere 5 hours; after the reaction stopped, cooled to room temperature, the mixture was poured into 150ml of water, and after standing for 4 hours, the aqueous phase was extracted with dichloromethane (60ml×2); the organic phases were combined, washed with water, and dried over anhydrous magnesium sulfate. The organic solvent was removed by vacuum rotary evaporation, and the crude product was purified by silica gel column chromatography (eluent-dichloromethane / petroleum ether 1:1 v / v) to obtain a dark red solid with metallic luster (yield 72%).
[0065] Mass Spectrum (MALDI-TOF-MS): m / z calculated 618.3; measured 618.4.
[0066] H NMR spectrum ( 1 H-NM...
Embodiment (3
[0068] Embodiment (3): N, N'-di-[9-(2'-ethyl-hexyl)-phenothiazine-vinyl]-succinonitrile (Dye3)
[0069] In a 100ml three-necked flask, put 3-formyl-9-(2'-ethyl-hexyl)-phenothiazine (42mmol) and diaminosuccinonitrile (20mmol) in about 40ml of glacial acetic acid, add A few drops of acetic anhydride were used as a catalyst, and heated to reflux at 120°C for 5 hours under nitrogen atmosphere; after the reaction stopped, cooled to room temperature, the mixture was poured into 150ml of water, and after standing for 4 hours, the aqueous phase was extracted with dichloromethane (60ml×2 ); the organic phases were combined, washed with water, and dried over anhydrous magnesium sulfate. The organic solvent was removed by vacuum rotary evaporation, and the crude product was purified by silica gel column chromatography (eluent-dichloromethane / petroleum ether 1:1 v / v) to obtain a dark red solid with metallic luster (yield 72%).
[0070] Mass Spectrum (MALDI-TOF-MS) m / z: Calculated 750.4; ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com