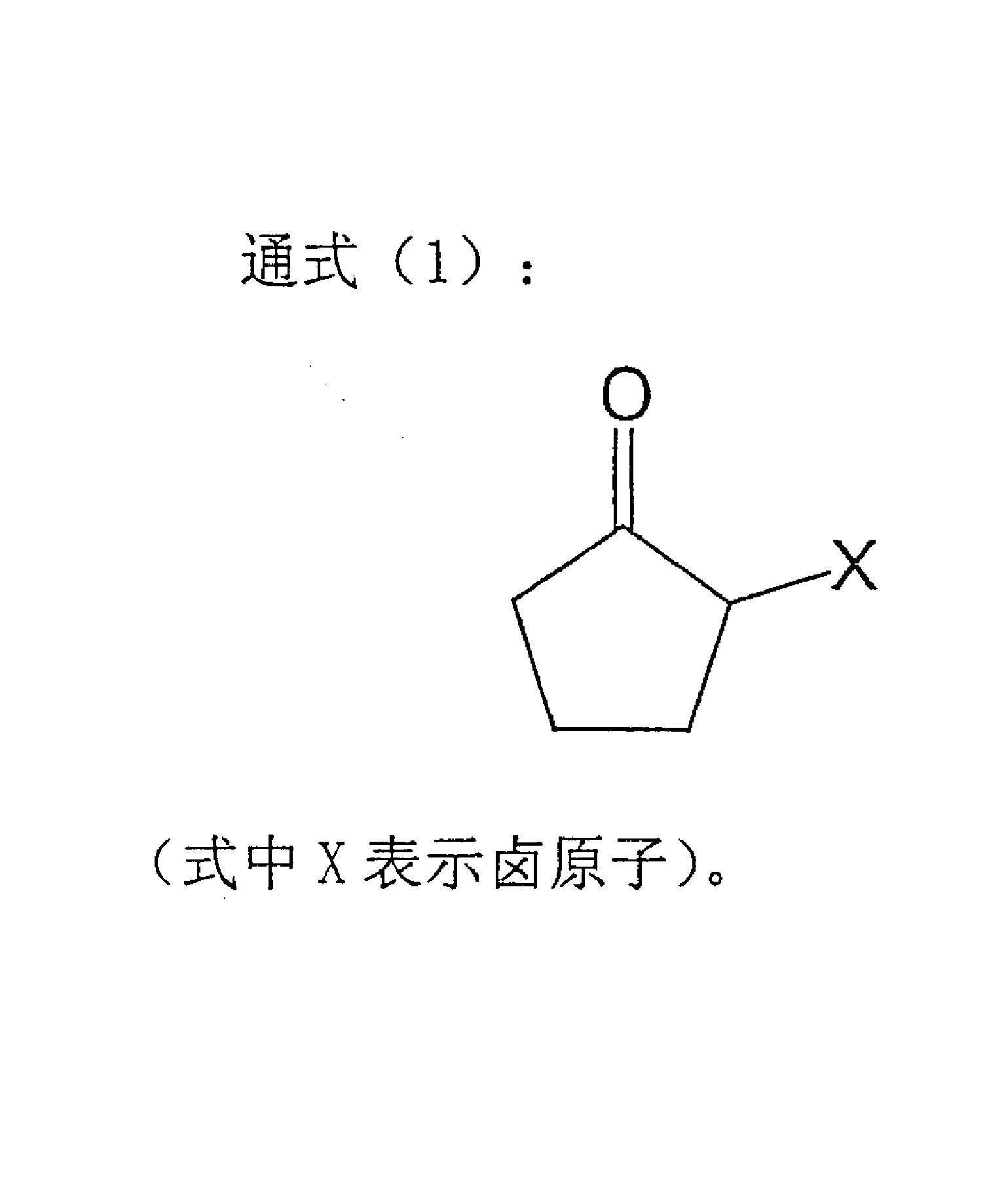
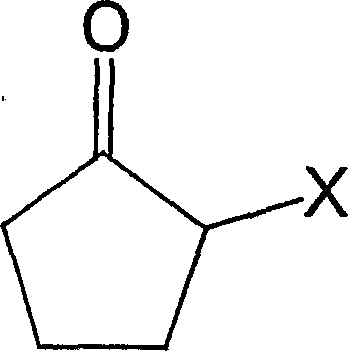
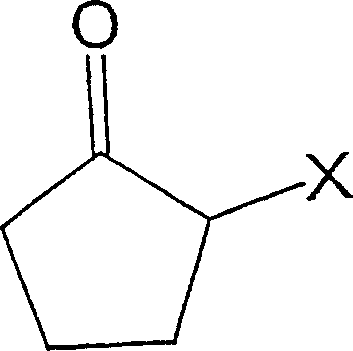
Process for producing 2-cyclopentene-1-one
A manufacturing method and technology of cyclopentene are applied in chemical instruments and methods, preparation of organic compounds, preparation of carbon-based compounds, etc., and can solve problems such as high boiling point and high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] To 2.2 parts by weight of N-methylpyrrolidone solvent, 0.01 parts by weight of lithium bromide monohydrate, 0.26 parts by weight of lithium carbonate, and 0.001 parts by weight of hydroquinone were added, and the temperature of the kettle was heated to 100°C. Thereto was added dropwise 1 part by weight of 2-bromocyclopentanone over 1 hour. After the dropwise addition was terminated, it was allowed to react for 1 hour, then the system was depressurized to 6.0-6.7 kPa, and the temperature of the kettle was distilled below 140° C. to obtain 2-cyclopenten-1-one with a yield of 58%.
Embodiment 2
[0041] To a mixed solvent of 1.1 parts by weight of N-methylpyrrolidone and 1.1 parts by weight of toluene, 0.26 parts by weight of lithium carbonate and 0.001 parts by weight of hydroquinone were added, and the temperature of the kettle was heated to 100°C. Thereto was added dropwise 1 part by weight of 2-bromocyclopentanone over 1 hour. After the dropwise addition was terminated, it was allowed to react for another 1 hour, then the system was depressurized to 6.0-6.7 kPa, concentrated at a kettle temperature below 70° C., and 90% of the toluene used was recovered. After concentration, reduce the pressure in the system to 6.0-6.7kPa, and distill at a kettle temperature below 140°C to obtain 2-cyclopenten-1-one with a yield of 51%.
Embodiment 3
[0043] To a mixed solvent of 1.1 parts by weight of N-methylpyrrolidone and 1.1 parts by weight of toluene, add 0.01 parts by weight of lithium bromide monohydrate, 0.26 parts by weight of lithium carbonate and 0.001 parts by weight of hydroquinone, and heat the temperature of the kettle to 100°C. Thereto was added dropwise 1 part by weight of 2-bromocyclopentanone over 1 hour. After the dropwise addition was terminated, it was allowed to react for another 1 hour, then the system was depressurized to 6.0-6.7 kPa, concentrated at a kettle temperature below 70° C., and 90% of the toluene used was recovered. After concentration, reduce the pressure in the system to 6.0-6.7kPa, and distill at the kettle temperature below 140°C to obtain 2-cyclopenten-1-one with a yield of 57%.
[0044] The effect of the invention
[0045] According to the present invention, it is possible to provide a method for producing 2-cyclopenten-1-one, which can efficiently synthesize the target 2-cyclopen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com