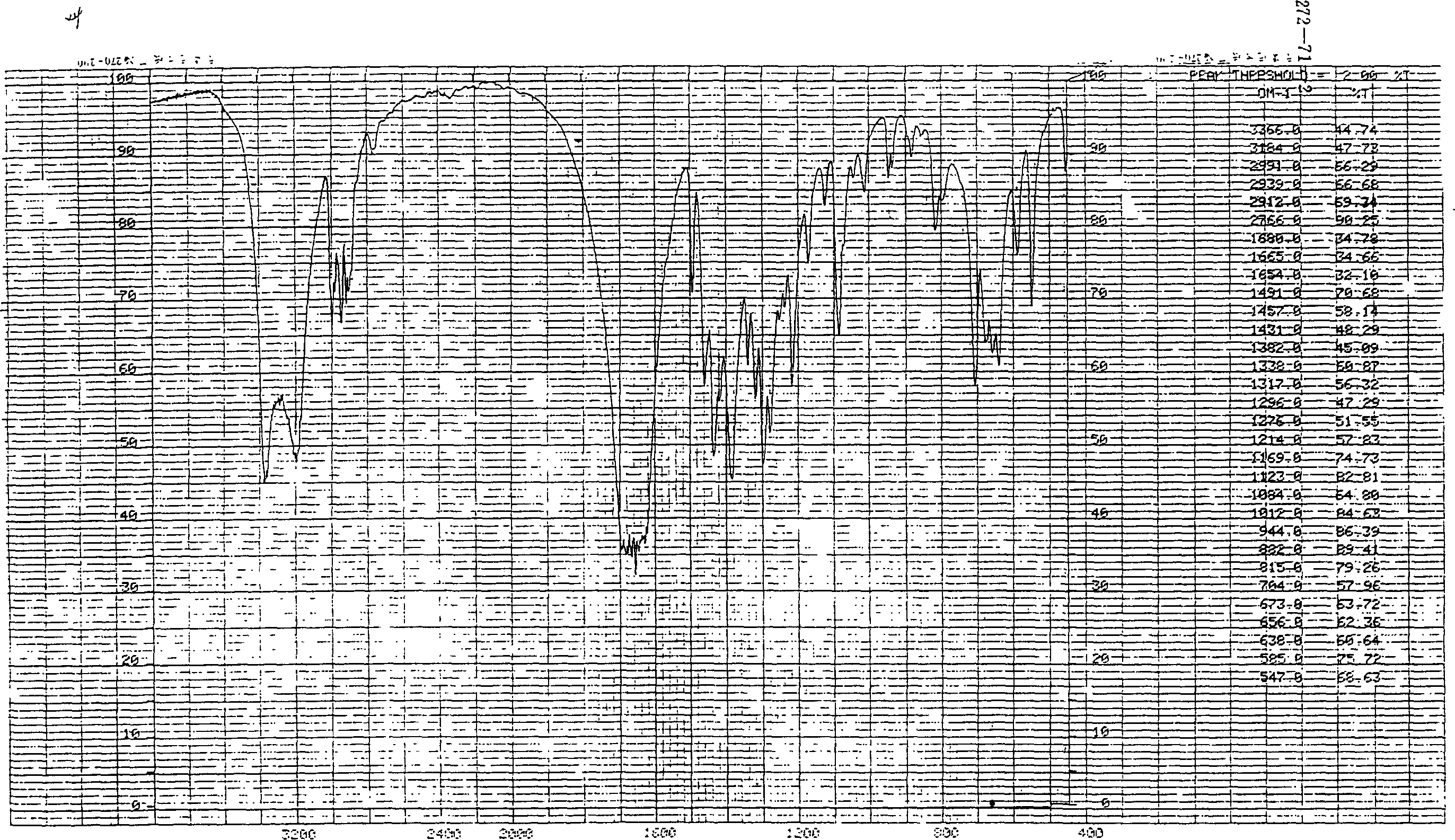
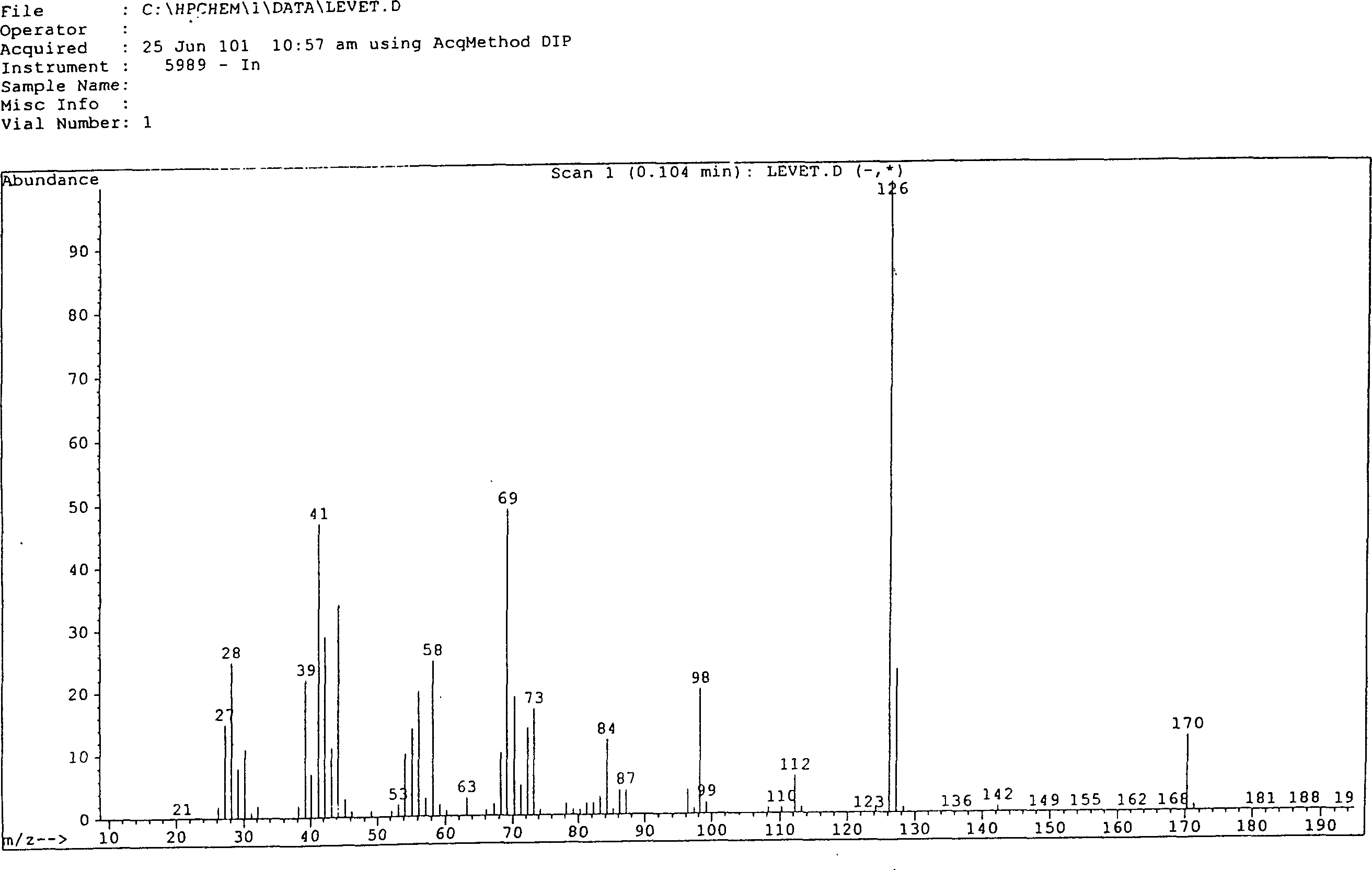
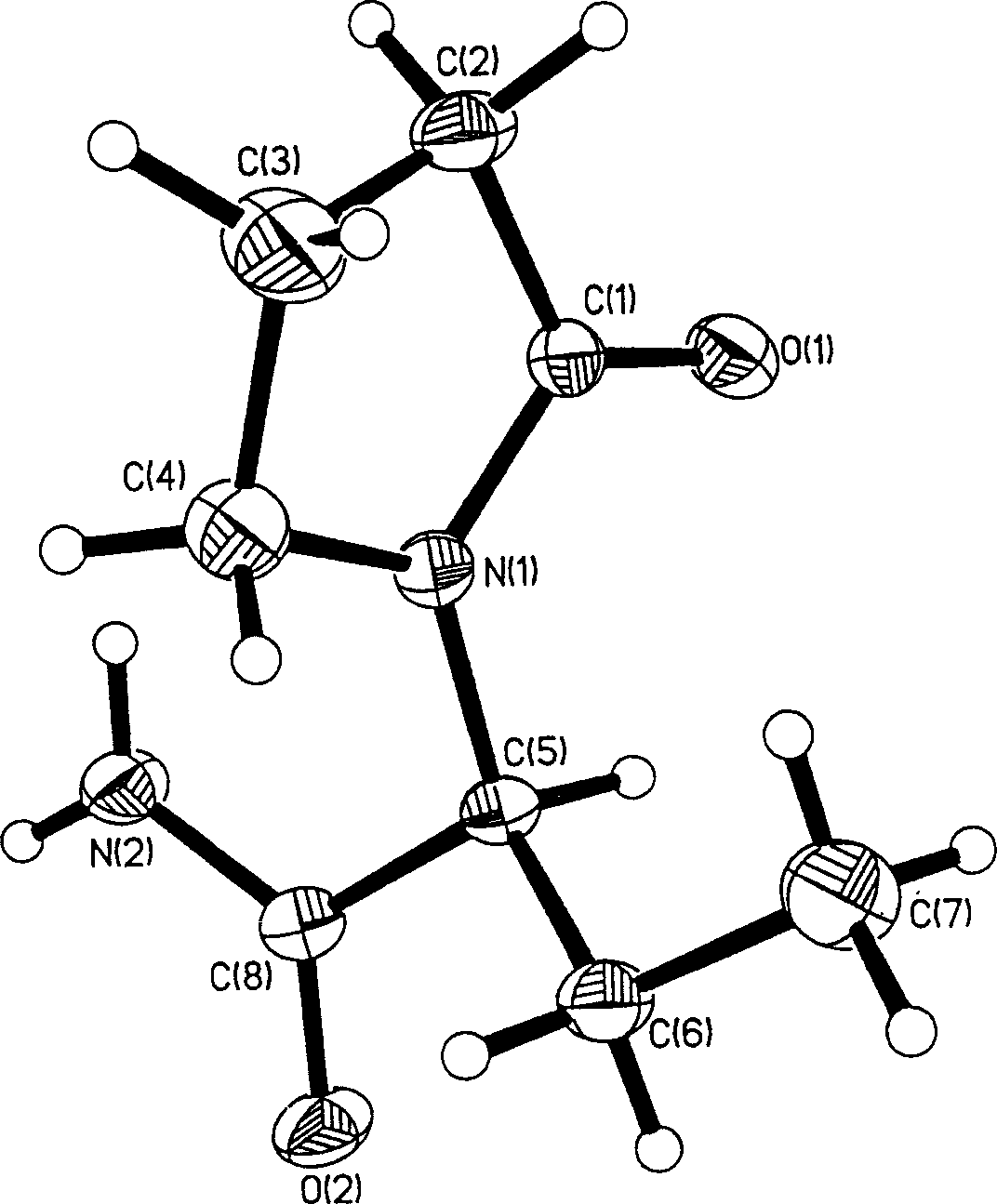
Synthesis of (S)-alpha-ethyl-2-oxi-1-pentazane acetamide
A synthetic process, aminobutyramide technology, applied in the direction of drug combination, neurological diseases, organic chemistry, etc., can solve the problems of unsuitable for industrial production, harsh reaction conditions, high cost of raw materials, etc., to achieve large-scale industrial production and simplify synthesis The effect of reaction steps and simplification of the degree of risk of operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Synthesis (II) of embodiment 1.α-amino-butyronitrile
[0026] In a 2L three-necked flask, add 63.7g (1.3mol) of sodium cyanide, 400ml of 20% ammonia water, and then add 78.7g (1.47mol) of ammonium chloride, and dissolve under stirring. 58g (1mol) of n-propionaldehyde solution was added dropwise, stirred at room temperature overnight, and extracted three times with 200ml of dichloromethane. Combine the organic extracts, add molecular sieves and dry. Concentration under reduced pressure gave about 60 g of crude α-amino-butyronitrile as a yellow oily liquid. Add hot ferrous sulfate solution to the aqueous solution, stir evenly to remove excess cyanide ions, and wash the used reaction flask, beaker and other containers with ferrous sulfate solution, and finally combine the waste water solution and pour it into the waste bucket.
Embodiment 2
[0027] Synthesis (II) of embodiment 2.α-amino-butyronitrile
[0028] Add 320g (6.2mol) of sodium cyanide, 402g (7.4mol) of ammonium chloride, and 2500ml of ammonia water into a 5L three-necked flask, stir until the solid dissolves, then add 290g (5mol) of propionaldehyde dropwise at room temperature, stir for 5 hours, and use dichloro Methane (2000ml) was extracted four times, the organic layers were combined, dried and the solvent was evaporated to obtain 416g of light yellow oil.
Embodiment 3
[0029] The preparation of embodiment 3.α-amino-butanamide hydrochloride (III)
[0030] 60 g (0.714 mol) of the crude α-amino-butyronitrile obtained in Example 1 was added to a three-necked flask, and 450 g (3.64 mol) of saturated hydrogen chloride isopropanol solution (30%) was added dropwise with stirring under ice cooling, and stirred overnight at room temperature. Raise the temperature to reflux, stir for 30 minutes, cool, and filter to obtain 70 g of light yellow crude product, mp 204°C. The crude product is added to a round-bottomed flask, 500ml of ethanol (95%) is added, heated to reflux, until fully dissolved, cooled, and white crystals are precipitated, filtered and dried to obtain 47.6g, mp222°C, content 99.3%, yield 40% (the first step , two-step combined calculation yield)
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com