Fusion expression method of mycobacterium tuberculosis ESAT-6 protein in pichia
A technology of mycobacterium tuberculosis, ESAT-6, applied in the field of biology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
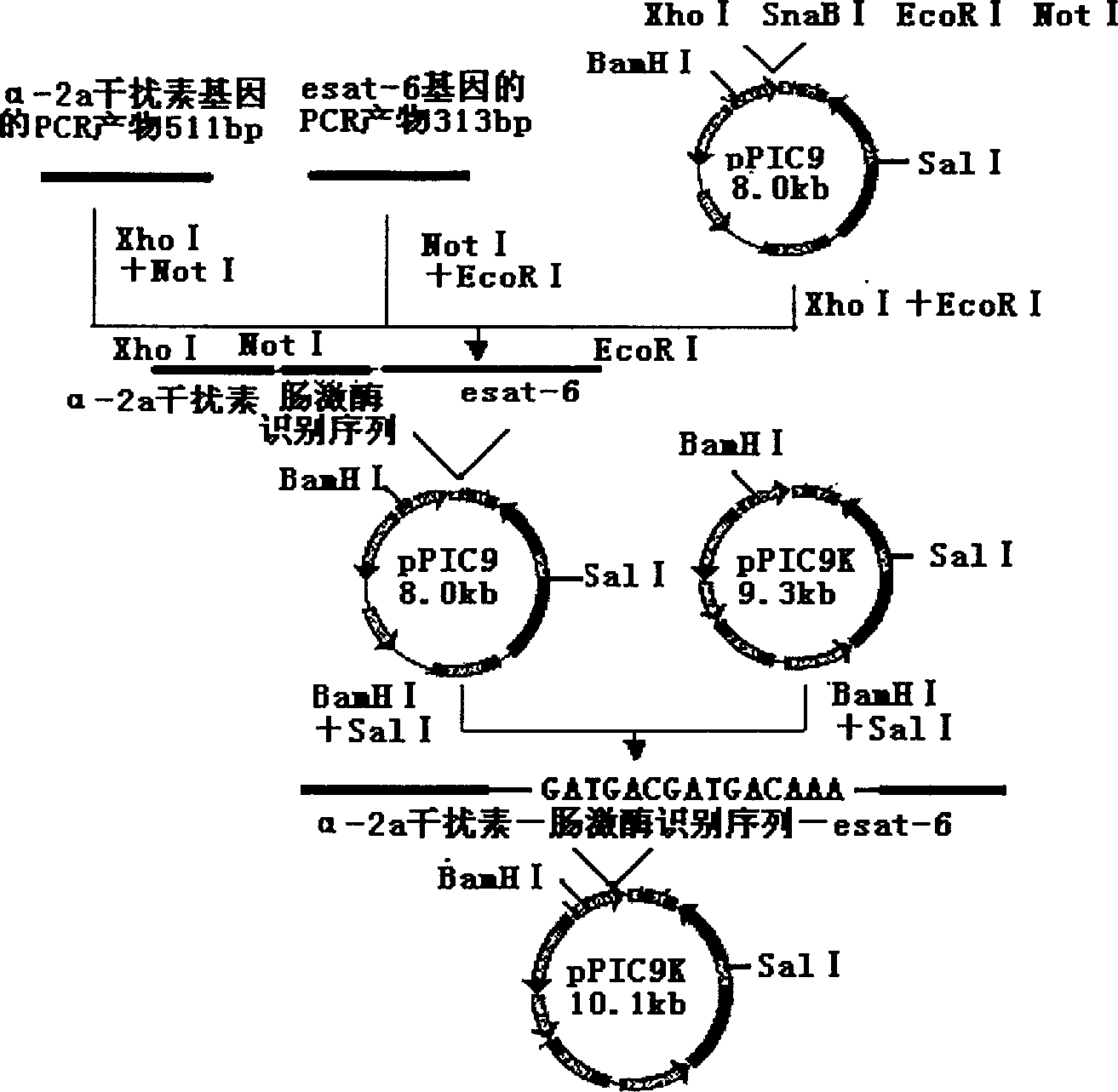
[0027] Example 1. Construction and identification of recombinant expression plasmids
[0028] The plasmid pPIC9 was double digested with XhoI and EcoRI, the PCR product containing the α-2a interferon gene was double digested with XhoI and NotI, and the PCR product containing the esat-6 gene was double digested with NotI and EcoRI. Then it was ligated with T4 DNA ligase, transformed into E. coli Top10 competent cells, and the recombinant plasmid pPIC9-α2a-esat6 was extracted and identified. Then the recombinant plasmid was double digested with BamHI and SalI to recover the small fragment, and the plasmid pPIC9K was also double digested with BamHI and SalI to recover the large fragment, ligated with T4 DNA ligase, transformed into E. coli Top10, and the recombinant expression plasmid pPIC9K- α2a-esat6, enzyme digestion identification and DNA sequence determination, proved that the construction of the recombinant expression plasmid was completely correct.
Embodiment 2
[0029] Example 2. Electrotransformation of Pichia pastoris SMD1168 with recombinant expression plasmids
[0030] Pichia pastoris SMD1168 (pep4, his4) was inoculated into YPD medium, shaken at 30°C to OD600 of 1.3-1.5, centrifuged at 5000 r / min at 4°C for 5 min, collected the cells, washed with pre-cooled sterile water and 1mol / L The cells were washed once with sorbitol, and finally suspended with 200 μl of 1 mol / L sorbitol to obtain SMD1168 competent cells. Take 80μl of SMD1168 competent cells and mix with 5μg of recombinant expression plasmid digested with SalI single enzyme, transform by electroporation under the conditions of 1300v, 25μF, 200Ω, suspend with 1ml of 1mol / L sorbitol, spread on RDB plate, and culture at 30°C for 3 days. ~4d, more than 1000 transformants were obtained.
Embodiment 3、G418
[0031] Example 3. Screening of G418 highly resistant yeast transformants
[0032] More than 1,000 yeast transformant colonies grown on RDB plates were seeded on YPD plates containing G418 concentrations of 1.5, 3.0, and 4.0 mg / ml, cultured at 30°C, and G418-resistant strains were screened step by step. As a result, 28 strains were obtained on a plate containing 4 mg / ml G418.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com