Detection of endometrial cancer
A technology for endometrial cancer and endometrium, which is applied in the field of endometrial cancer detection and can solve problems such as undetected
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
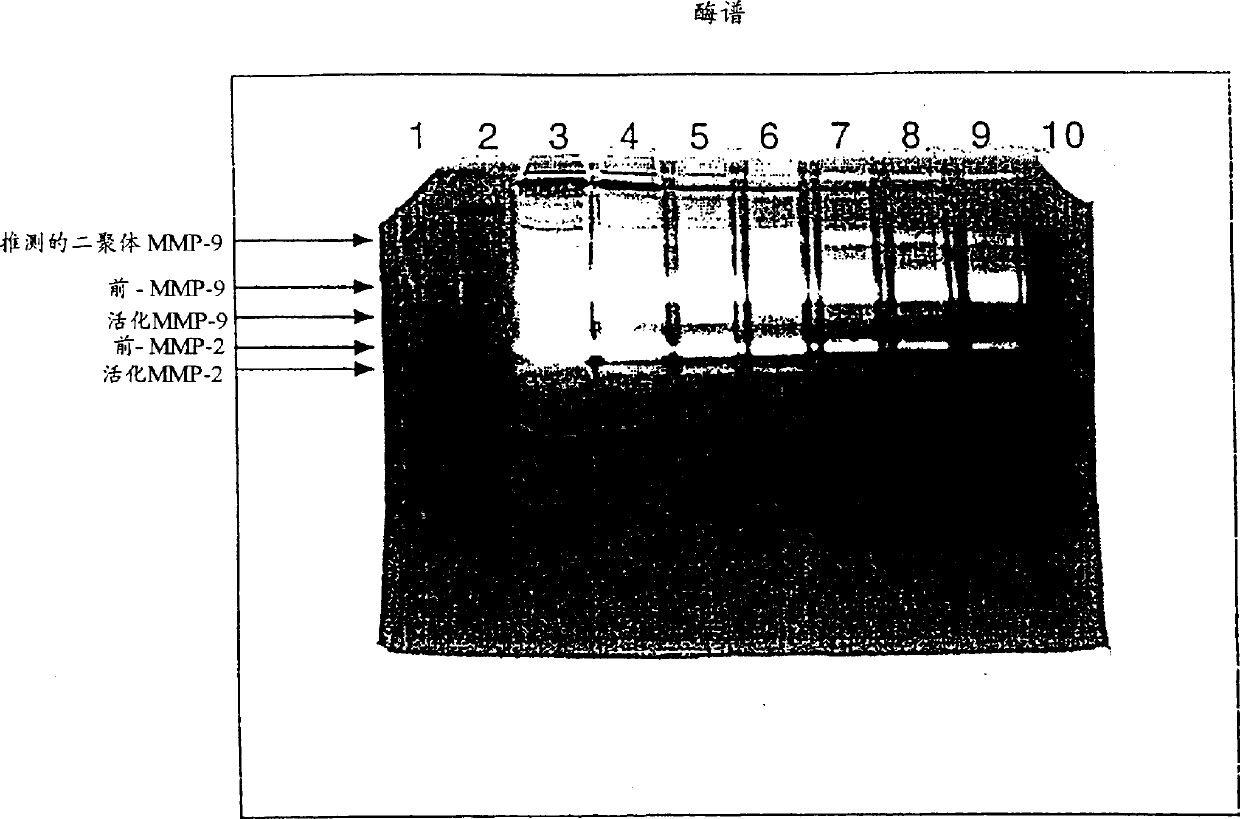
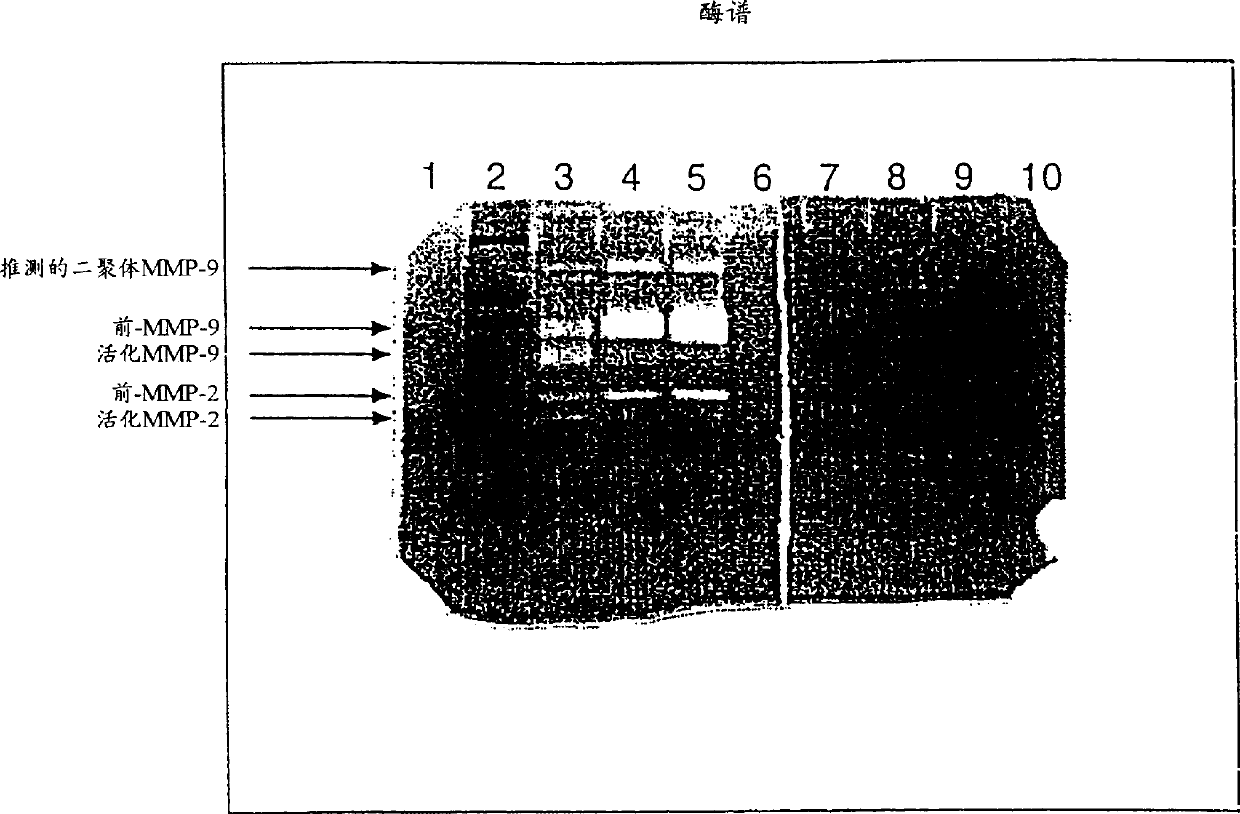
[0077] Example 1 Release of MMPs into the Uterine Cavity of Patients Undergoing Endometrial Cancer Study
[0078] Samples from 52 endometrial cancer patients and 40 control patients undergoing diagnostic procedures for uterine bleeding or other gynecological diseases were examined for MMP-2 and MMP-9 by zymography and by image cytometry DNA ploidy of endometrial surface cells. Patients found to have endometrial cancer were assessed for grade of malignancy. MMP-2 and MMP-9 were further divided into the following hypothetical assay types: latent MMP-2, active MMP-2, dimeric MMP-9, preglycosylated MMP-9 and activated MMP-9. The identification of latent and active MMP-2 and MMP-9 was subsequently determined as described in the Examples below.
[0079] The results are summarized in Tables 1 and 2. In addition, MMP-1, MMP-3, and MMP-7 were measured by casein zymography, but no detectable levels were found in either controls or uterine washings from patients with endometrial cance...
Embodiment 2
[0098] Example 2 ELISA analysis of MMP-9 and MMP-2
[0099] Biotrak TM MMP-9 ELISA Kit (Calbiochem R), capable of providing simple and specific detection of MMP-9 in uterine wash samples. The assay has a sensitivity of 0.6 ng / ml. This assay uses two antibodies directed against different epitopes of MMP-9. All MMP-9 present in the samples or standards was bound to microtiter plates previously coated with anti-MMP-9. This is followed by a second incubation step in which a detection antibody conjugated to horseradish peroxidase is added to form an immobilized complex. Tetramethylbenzidine substrate was added to each well, and a color reaction was performed to detect the amount of peroxidase complex in each well. The blue color of the product was measured at 630 nm using a microtiter plate spectrophotometer (Beckman). Prepare a standard curve by plotting the average optical density at 630 nm against the standard ng / ml. The concentration (ng / ml) of each sample was determine...
Embodiment 3
[0102] Example 3 Cell Biology Research
[0103] Hysterectomy is part of the treatment for patients who have been diagnosed with uterine cancer. After hysterectomy, endometrial tissue is removed, if possible, from the following two locations:
[0104] (a) corresponding to the location of the malignant lesion,
[0105] (b) On microscopic examination, the endometrium appears to be in a normal position.
[0106] Both tumor tissue and normal tissue were in the presence of 0.15M NaCl, 10mM CaCl 2 , and 0.05% Brij35 in 50 mM Tris-HCl buffer (pH 7.5), and homogenate on ice. The supernatant was obtained by centrifugation, and the protein concentration was determined by the dye binding method with BilRad protein assay reagent.
[0107] Examination of MMP phenotypes in tissue homogenates by gelatin zymography. Supernatants, calibrated to have the same amount of protein in each lane, were poured into gels with gelatin and subjected to SDS-PAGE as above.
[0108] Zymograms showed rel...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com