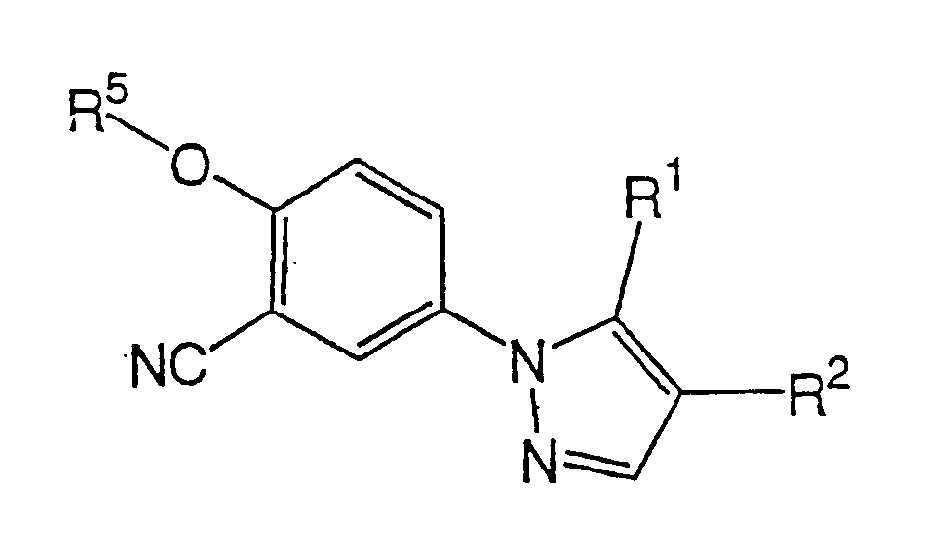
1-phenylpyrazole compounds and medicinal application thereof
A technology of phenylpyrazoles and compounds, applied in the field of new 1-phenylpyrazole compounds, which can solve problems such as the practical application of substances that have not yet realized the generation of active oxygen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Add ethyl 2-cyano-3-ethoxyacrylate to the ethanol solution (50 ml) of 3-cyano-4-isobutoxyphenylhydrazine (5 g) obtained in Starting Material Synthesis Example 1 under stirring (4.2 g) and the mixture was heated to reflux for 2 hours. After cooling, the precipitated crystals were collected by filtration to obtain 5.1 g of ethyl 5-amino-1-(3-cyano-4-isobutoxyphenyl)pyrazole-4-carboxylate, melting point 115-117°C. Example 2
Embodiment 2
[0066] To a solution of ethyl 5-amino-1-(3-cyano-4-isobutoxyphenyl)pyrazole-4-carboxylate (1 g) in ethanol (10 ml) was added 1 ml of 5N aqueous hydrochloric acid under stirring, The mixture was refluxed for 2 hours with heating. After the reaction was completed, the reaction mixture was poured into water, and the mixture was neutralized with acetic acid. Precipitated crystals were recrystallized from a mixed solvent of dioxane and water to obtain 0.4 g of 5-amino-1-(3-cyano-4-isobutoxyphenyl)pyrazole-4-carboxylic acid, m.p. 204°C (decomposition). Example 3
Embodiment 3
[0067] Subsequently, ethyl 5-amino-1-(3-cyano-4-isobutoxyphenyl)pyrazole-4-carboxylate (1.64 g) obtained in Example 1 was dissolved in tetrahydrofuran under stirring ( 16 ml) was added isoamyl nitrite (1.75 g), and the mixture was heated to reflux for 1.5 hours. After completion of the reaction, the solvent was evaporated under reduced pressure, and the resulting residue was recrystallized from ethanol to obtain 1.38 g of ethyl 1-(3-cyano-4-isobutoxyphenyl)pyrazole-4-carboxylate, melting point 138 -139°C. Example 4
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com