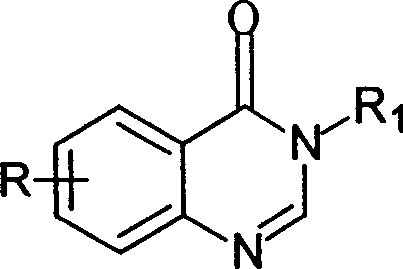
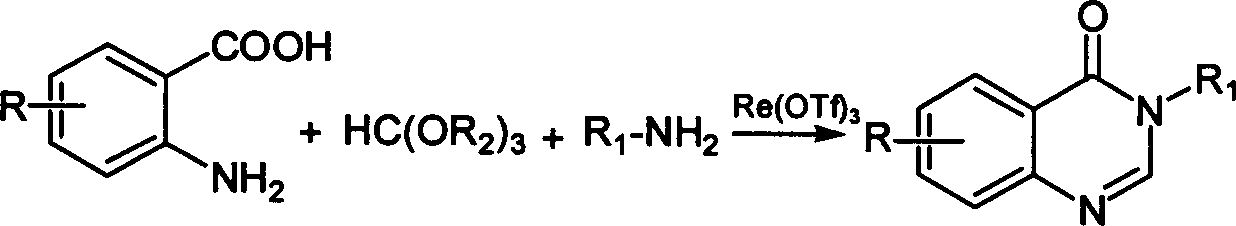Synthesis of quinazoline-4 (3H) derivative
A synthesis method and derivative technology, applied in the direction of organic chemistry, etc., can solve the problems of low yield, long reaction time and high reaction temperature, and achieve the effects of high yield, simple method and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Get anthranilic acid (10mmol, 1.37g), formic acid (13mmol, 0.78g) and aniline (13mmol, 1.41g) and catalyst Yb (OTf) 3 (0.5mmol, 62mg) were placed in a reaction flask and mixed together, heated and stirred, the temperature was controlled at 50°C, and the reaction was carried out for 60 minutes. The TLC was followed to complete the reaction. Then, water was added to quench the reaction, extracted three times with ethyl acetate, and the organic layers were combined. use Na 2 SO 4 Drying, rotary evaporation to remove the solvent, and recrystallization with ethanol to obtain the corresponding product, 1.64 g, yield 74%.
[0027] mp138-139°C, 1 H-NMR (500Hz, CDCl 3): δ8.32(d, 1H), 8.13(s, 1H), 7.60-7.72(m, J=27.29Hz, 2H), 7.44(t, J=14.34Hz, 1H), 7.23-7.31(m, J=38.12Hz, 5H); 13 C NMR (50MHz, CDCl 3 )δ160.5, 147.5, 146.4, 135.6, 134.3, 129.1, 128.3, 128.1, 127.1, 126.6, 127.2, 127.0, 122.1, 49.7; GC / MS: M + = 222.1; Anal. Calcd for C 14 h 10 N 2 OC, 75.66; H, 4.54; N,...
Embodiment 2
[0029] Get anthranilic acid (10mmol, 1.37g), trimethyl orthoformate (13mmol, 1.92g) and p-methylaniline (13mmol, 1.41g) and catalyst Yb (OTf) 3 (0.5mmol, 62mg) were mixed together in a reaction flask, heated and stirred, the temperature was controlled at 50°C, and the reaction was carried out for 10 minutes. The TLC was followed to complete the reaction. Then, water was added to quench the reaction, extracted three times with ethyl acetate, and the organic layers were combined. use Na 2 SO 4 Drying, rotary evaporation to remove the solvent, and recrystallization with ethanol to obtain the corresponding product, 2.31 g, yield 98%.
[0030] mp144-145°C, 1 H-NMR (500Hz, CDCl 3 ): δ8.22(d, 1H), 8.12(s, 1H), 7.61-7.71(m, J=24.23Hz, 2H), 7.42(t, J=13.31Hz, 1H), 7.15(d, J =8.6Hz, 2H), 6.88(d, J=8.5Hz, 2H), 2.33(s, 3H). 13 C NMR (50MHz, CDCl 3 )δ161.5, 144.5, 146.4, 135.3, 134.3, 129.0, 128.2, 128.1, 127.0, 126.5, 127.1, 127.0, 122.0, 49.6; GC / MS: M + =236; Anal. Calcd for C 1...
Embodiment 3
[0032] Catalyst Yb(OTf) 3 reuse of
[0033] Get anthranilic acid (10mmol, 1.37g), triethyl orthoformate (13mmol, 1.92g) and aniline (13mmol, 1.41g) and catalyst Yb (OTf) 3 (0.5mmol, 62mg) were mixed together in a reaction flask, heated and stirred, the temperature was controlled at 50°C, and the reaction was carried out for 10 minutes. The TLC was followed to complete the reaction. Then, water was added to quench the reaction, extracted three times with ethyl acetate, and the organic layers were combined. use Na 2 SO 4 Drying, rotary evaporation to remove the solvent, and recrystallization with ethanol to obtain the corresponding product, 2.17 g, yield 98%.
[0034] Catalyst Yb(OTf) 3 Stay in the water layer, remove the water by heating, then heat and vacuum at 100°C for 2-8 hours, and the catalyst is reused.
[0035] Under the same reaction conditions:
[0036] Catalyst Yb(OTf) 3 The first repetition yielded 2.15 g, a 97% yield.
[0037] Catalyst Yb(OTf) 3 The second...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com