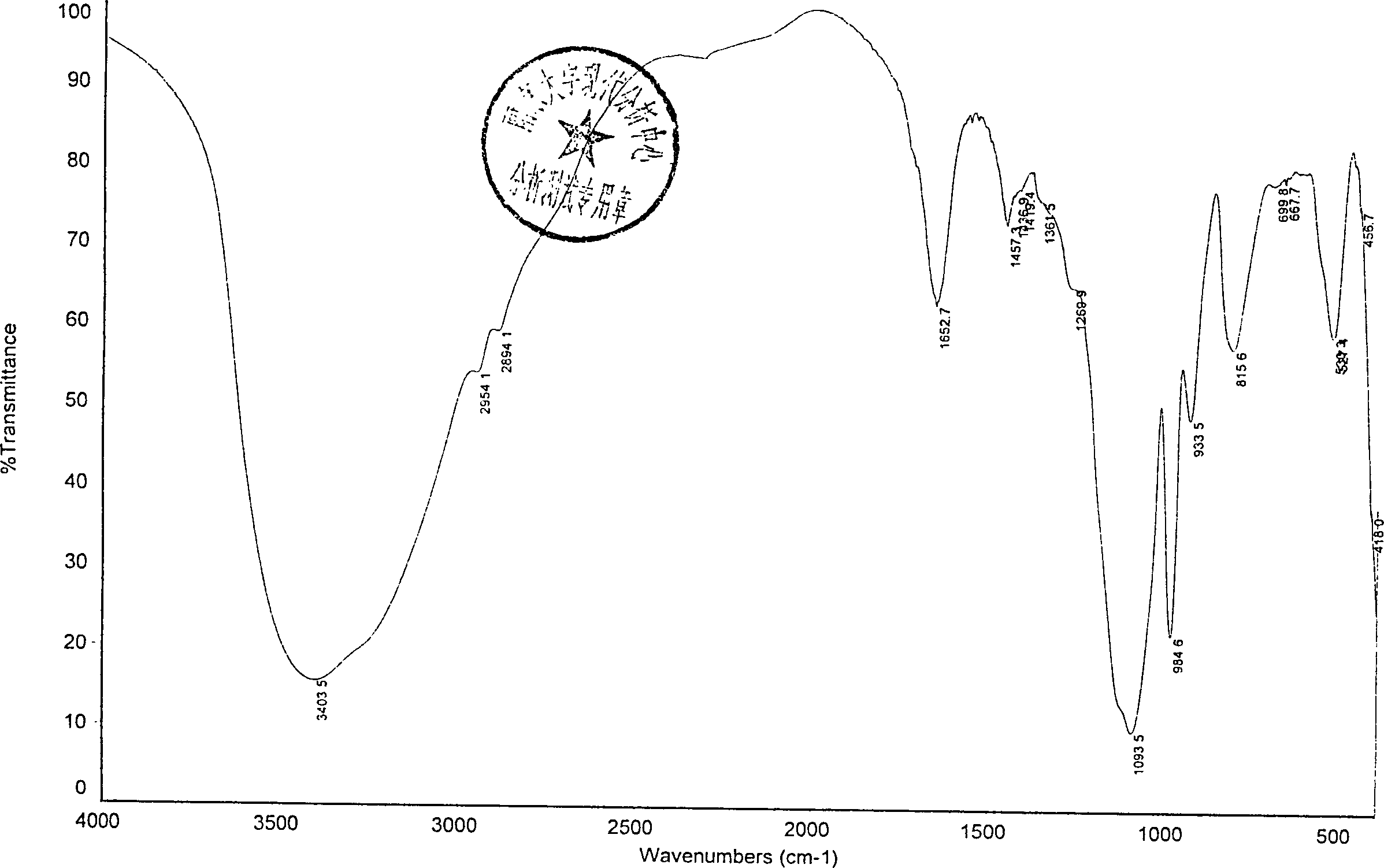
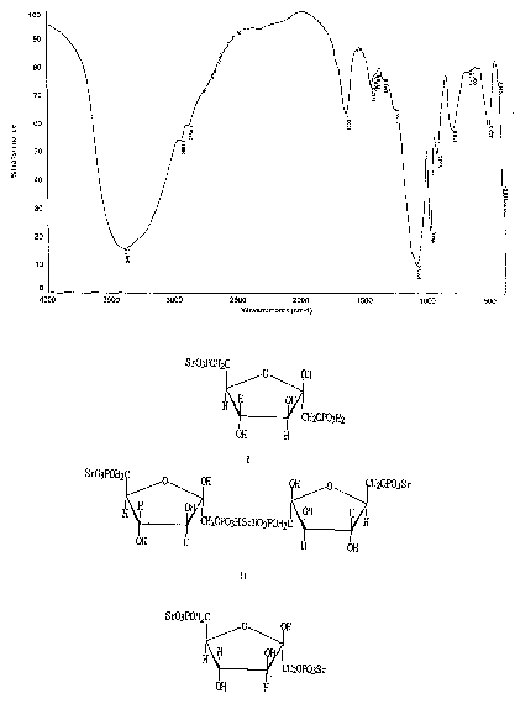
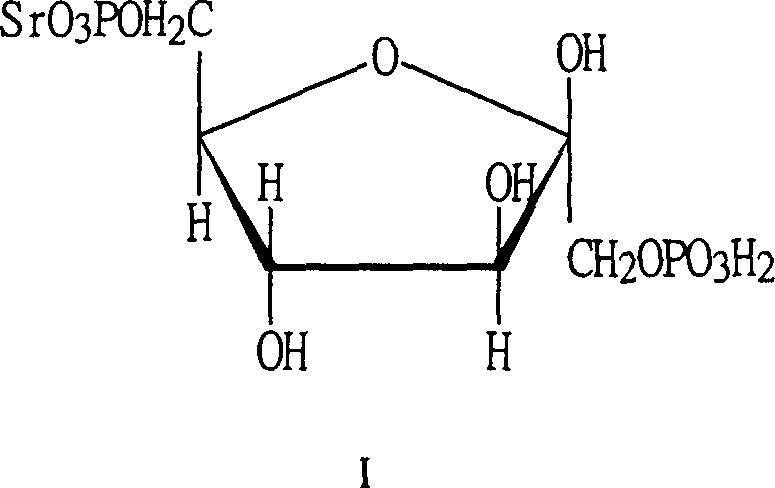
1,6-fructostrontium biphosphate compounds and their preparing process and medical application
A technology of strontium salt compound and fructose diphosphate, applied in 1 field, can solve the problem of huge therapeutic dose
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Method used
Image
Examples
example 1
[0061] Dissolve 1.2kg of glucose, 0.4kg of sodium dihydrogen phosphate, 0.9kg of disodium hydrogen phosphate, and 0.1kg of magnesium chloride in 10L of water, heat to 37°C, add 1kg of brewer’s yeast and 600ml of toluene, stir at 37°C for 3.5 hours, and use 6N Adjust the pH to 2.0-2.5 with hydrochloric acid to stop the reaction, filter, dilute the filtrate 5 times with distilled water, adsorb on anion exchange resin HD-30, wash with 0.02N hydrochloric acid until the phosphate content of FDP is less than 0.05g / L, then use 0.5N hydrochloric acid to elute When the FDP content is less than 1g / L, collect 22L of eluate, FDPH 4 The concentration is 24g / L, and the eluate is dechlorinated with a nanofiltration membrane, concentrated, and the concentrated solution is collected. The FDP content in the concentrated solution is measured to be 86g / L, and the volume is 5.8L. Add 452g of strontium acetate and stir for 30 minutes. , concentrated in vacuo, added 300g of medicinal activated carbo...
example 2
[0071] Dissolve 1.2kg of glucose, 0.4kg of sodium dihydrogen phosphate, 0.9kg of disodium hydrogen phosphate, 0.1kg of magnesium chloride, 0.025Kg of ammonium chloride, and 0.04Kg of potassium chloride in 10L of water, heat to 37°C, add 1kg of brewer’s yeast and 600ml of toluene, stirred at 37°C for 3.5 hours, adjusted the pH to 2.0-2.5 with 6N hydrochloric acid to stop the reaction, filtered, diluted the filtrate 5 times with distilled water, adsorbed on anion exchange resin HD-30, washed with 0.02N hydrochloric acid until the phosphate content of FDP was less than 0.05g / L, use 1N sodium chloride to elute until the FDP content is less than 1g / L, collect 23L of the eluate, the concentration is 22.8g / L, concentrate in vacuo, add 300g of activated carbon for decolorization, add 2 times the volume of the solution in alcohol for desalination , suction filtration, the filtrate was washed 3 times with 0.5 times the volume of 75% alcoholic water, poured off the upper alcohol aqueous s...
example 3
[0079] Dissolve 120g of glucose, 40g of sodium dihydrogen phosphate, 90g of disodium hydrogen phosphate, and 10g of magnesium chloride in 1L of water, heat to 37°C, add 100g of brewer’s yeast and 60ml of toluene, stir at 37°C for 3.5 hours, and adjust the pH to 2.0-2.5 Stop the reaction, filter, dilute the filtrate 5 times with distilled water, adsorb on anion exchange resin HD-30, wash with 0.02N hydrochloric acid until the phosphate content of FDP is less than 0.05g / L, then use 0.5N hydrochloric acid to elute until the FDP content is less than 1g / L, the eluate was filtered with a nanofiltration membrane with a molecular weight cut-off of 200, and the macromolecular solution was collected. The FDP content in the filtrate was measured to be 92g / l, and the volume was 574ml. Add 30.1 g of strontium acetate to this solution, stir and react for 4 hours, add 2.5 times the volume of alcohol in the solution, and stir for 15 minutes, vacuum filter, wash with a small amount of 80% alcoh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com