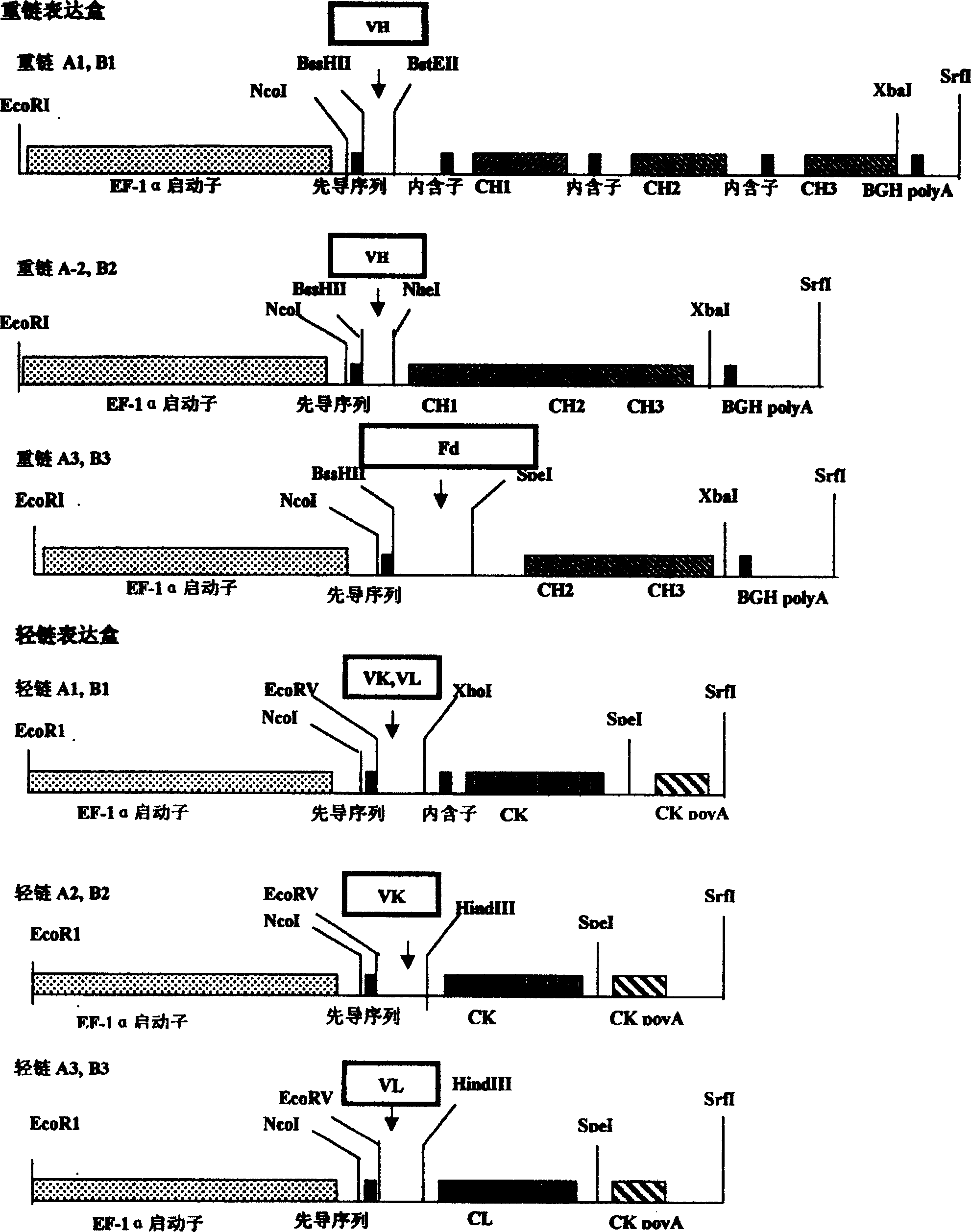
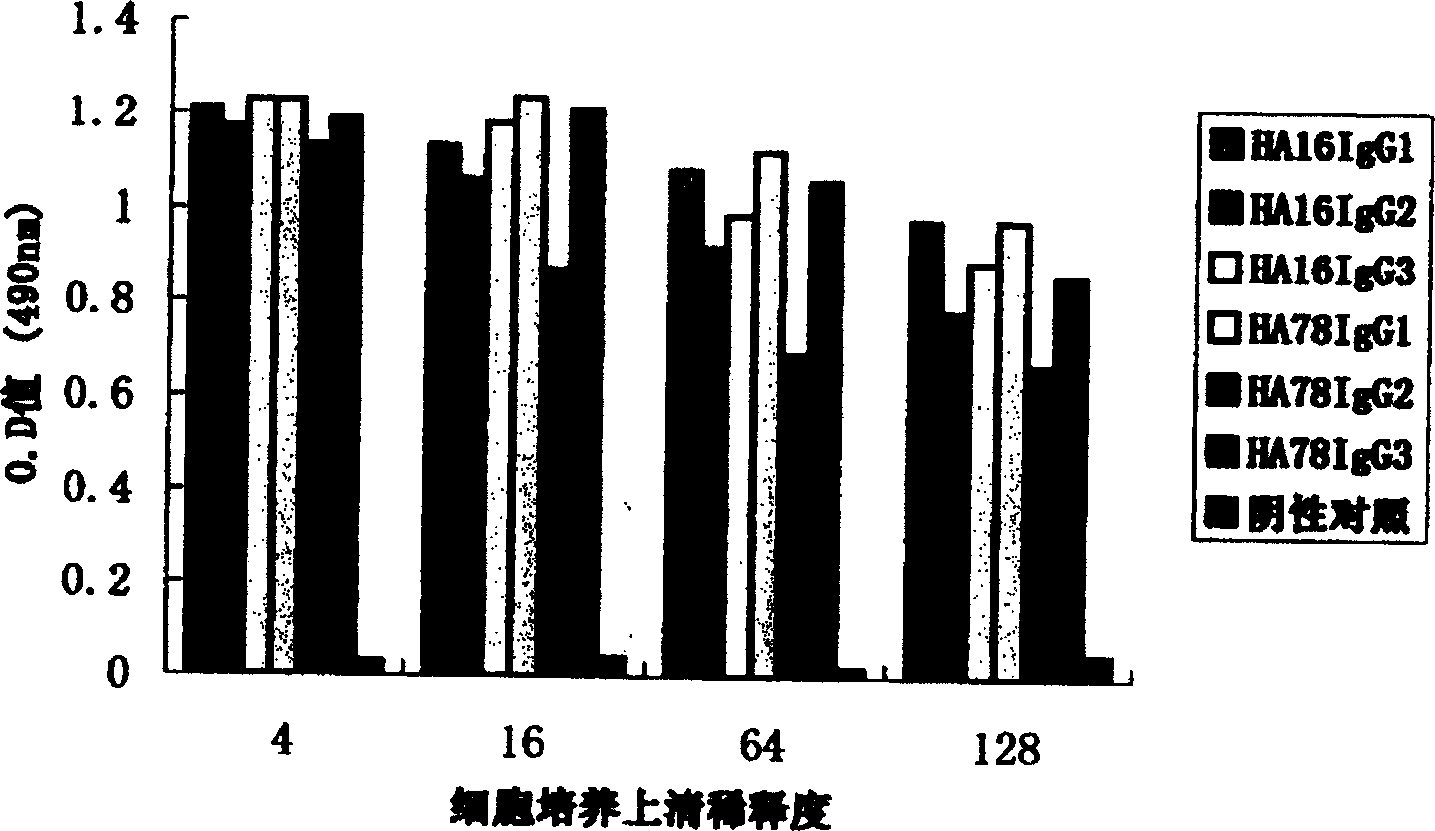
A group of efficient expression carriers for humanized antibody of mammal cell
An expression vector and high-efficiency expression technology, which is applied in the field of high-efficiency expression vectors for a group of mammalian cell human antibodies for production, and can solve the problem of no commercial vectors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0057] Example 1: The primers used in vector construction and application were designed by ourselves and synthesized in MWG (Germany) commercial company. The sequence numbers of all primers are as follows:
[0058] 1. BssHII-F
[0059] 5′ TATCCGCGCGCACTCCCCAGGTGCAACTGCTCGAGTCTGGG-3′
[0060] 2. XbaI-R
[0061] 5′-TGCTCTAGATTATTTTCCCGGACTTAAGGAGAG-3′
[0062] 3. BstEII-HR
[0063] 5′-GGAGACGGTGACCAGGGTTCCCTC-3′
[0064] 4. XhoI-KR
[0065] 5′-CTTGATCTCGAGCTTGGTCCCCTGGTC-3′
[0066] 5. Hind III R
[0067] 5′-CGCAAGCTTATTGAATCAAAGGATATATGAC-3′
[0068] 6. XohI-LR
[0069] 5′-TAGGACCTCGAGCTTGGTCCCCTCCGCC-3′
[0070] 7. SpeI-LR
[0071] 5′-GGACTAGTCCTATGAACATTCTGTAGG-3′
[0072] 8. SpeI-KR
[0073] 5′-GGACTAGTCCTAACACTTCTCCCCTGTTGA-3′
[0074] 9. EcoRV-VL2
[0075] 5′-CCGGATATCGCCCTCACGCAGCCTGCCTCCGTG-3′
[0076] 10. EcoRV-VL5
[0077] 5′-CCGGATATCGTG-CTCACGCCGCCCTCAGTG-3′
[0078] 11. EcoRV-VK1a
[0079] 5′-CCGGATATCGTGCTCACCCAGTCTCCA-3′ ...
example 2
[0082] Example 2: Obtaining the elements of the antibody expression cassette in the vector system: 1. The main elements of the backbone vector EF-1a, DHFR gene and IRES gene are from vectors EF-VH, EF-VL, pSV2-DHFR and IRES respectively. 2. The antibody light and heavy chain constant region genes in the vectors VH-dhfr1, VH-IRES-shfr1, VK-dhfr1, and VH-IRES-dhfr1 are from the vectors pTA-IgGH and pTA-K developed by the inventor Professor Bautz's laboratory. 3. The antibody genes in the vectors VH-dhfr2, VH-dhfr3, VH-IRES-dhfr2, VH-IRES-dhfr3, VK-dhfr2, VK-dhfr3, VH-IRES-dhfr2, VH-IRES-dhfr3 are from the inventor Liang Mi Fang and Professor Bautz's laboratory jointly developed vectors pAc-K-CH3, pAc-K-Fc and pAc-L-CH3.
example 3
[0083] Example 3. Construction of expression vector: as attached figure 1 And attached figure 2 , is a schematic diagram of the backbone vector and different expression cassettes of the series of vectors in the present invention, which mainly consists of the following key steps:
[0084]1) Use BssHII and XbaI double enzymes and the carrier EF-VH, electrophoresis to recover the carrier band, this vector contains the EF-1a promoter and BGHpolyA tail and the new enzyme gene (NEO), about 5468 base pairs (bp),
[0085] 2) Using the carrier pTA-IgGH as a template, use primers BssHII-F and XbaI-R to amplify the heavy chain constant region gene containing the intron gene by PCR method, and recover the fragment by electrophoresis, about 1796bp, containing the VH gene cloning site BssHII and BstEII.
[0086] 3) Using the vector pAc-K-CH3 as a template, use primers BssHII-F and XbaI-R to amplify the heavy chain constant region gene without intron gene by PCR method, and recover the fr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com