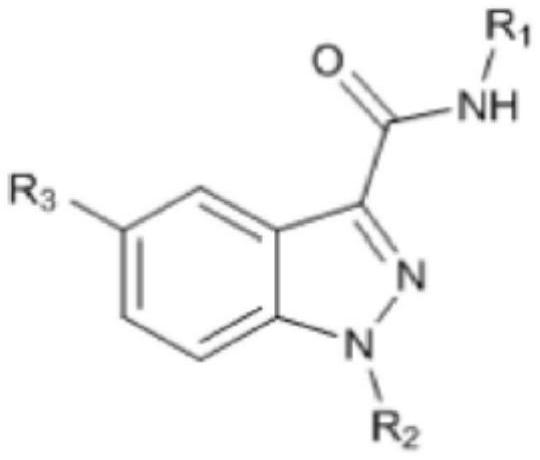
Novel indazole derivatives and uses thereof
A compound, C4-C12 technology, applied in the field of indazole derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0133] Example 1. Synthesis of Indazole Derivatives and Confirmation of Physicochemical Properties
[0134] The synthetic procedures and physicochemical properties of the compounds 1 to 73 of the present invention are as follows.
[0135] Compound 1: tert-butyl 4-(5-(2-fluoropyridin-3-yl)-1H-indazole-3-carboxamido)piperidine-1-carboxylate
[0136] [Reaction 1]
[0137]
[0138] 1) Synthesis of 5-bromo-1H-indazole-3-carboxylic acid (5-bromo-1H-indazole-3-carboxylic acid)
[0139] A suspension of Indazole-3-carboxylic acid (500 mg, 3.08 mmol) in acetic acid (AcOH) (25 ml) was heated to 120°C to dissolve the starting material. The reaction mixture was cooled to 90°C, and the reaction mixture to which bromine (0.32 ml, 6.16 mmol) was added was stirred at 90°C for 18 hours. The reaction mixture was diluted with water (20 ml) and stirred for 1 hour. The solid formed in the reaction mixture was filtered and sucked dry to give compound 15-246 (550 mg, 2.28 mmol, 74%) as a white...
Embodiment 2
[0731] Example 2. Confirmation of TRIB2 or YAP inhibitory activity
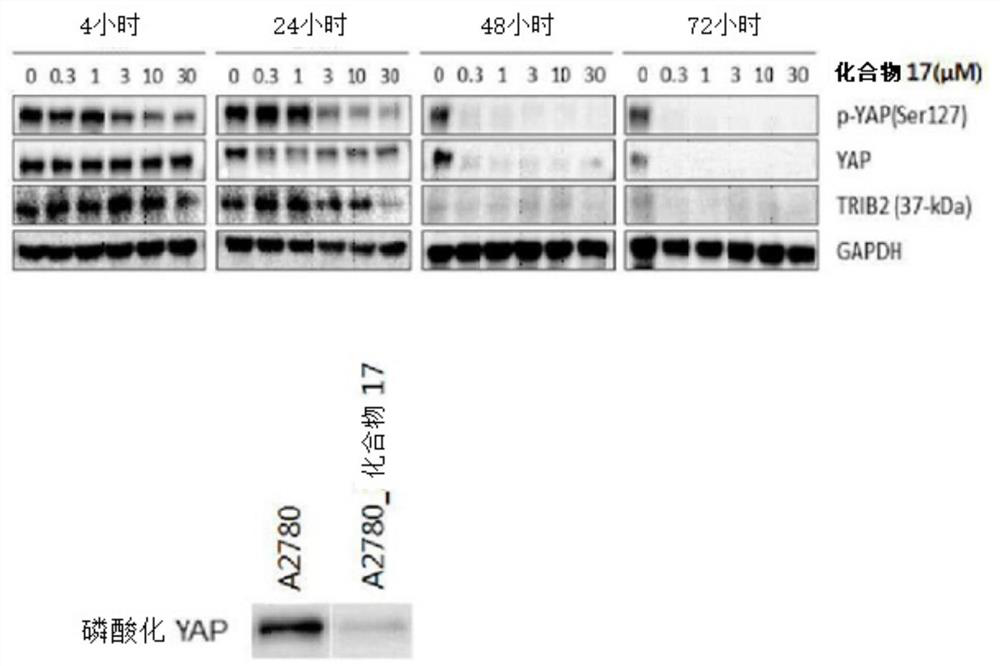
[0732] Using the compounds 1 to 73 of the present invention synthesized from the above-mentioned Example 1, the TRIB2 kinase inhibitory activity and the cancer cell proliferation inhibitory activity were confirmed as shown in the following Table 1, and the YAP inhibitory activity was as follows figure 1 shown.
[0733] The TRIB2 kinase activity assay was commissioned by eurofins. The simple method is as follows: First, mix human TRIB2 enzyme with 8mM 3-morpholinopropanesulfonic acid (MOPS) (pH 7.0) , 0.2mM ethylenediaminetetraacetic acid (EDTA), 250μM substrate (RRRFRPASPLRGPPK), using 10mM Magnesium acetate (Magnesium acetate), 15μM microorganism γ-33P-ATP (gamma-33P-ATP), Different concentrations of test drugs were treated and allowed to react at room temperature for 120 minutes. Subsequently, the reaction was terminated by treatment with 0.5% phosphoric acid, and 10 μL of the reaction solution was droppe...
preparation example 1
[0741]
[0742] Mix 2 g of the present compound 17 (5-(2-fluoropyridin-3-yl)-N-(pyridin-4-yl)-1H-indazole-3-carboxamide), 1 g of lactose, and fill to gas The powder is prepared in a sealed bag.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com