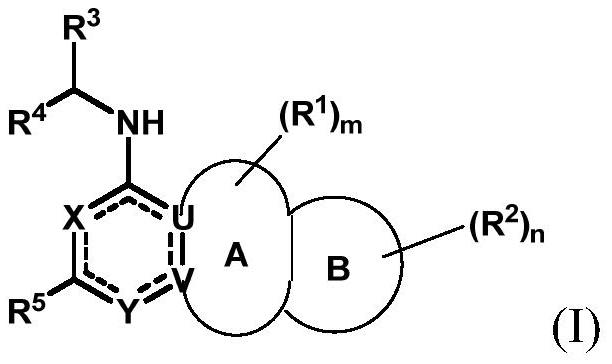
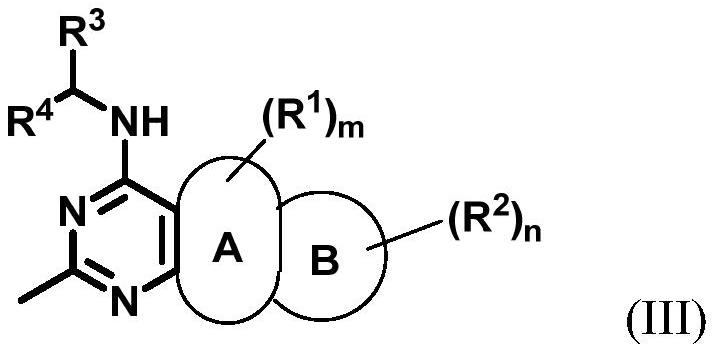
Substituted bicyclic aromatic heterocyclic amine inhibitor as well as preparation method and application thereof
An unsubstituted, heterocyclic-based technology, applied in the field of substituted bicyclic and aromatic heterocyclic amine inhibitors and its preparation, can solve the problems of reducing tumor cell proliferation rate and survival rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0223] The present invention also provides a preparation method of a pharmaceutical composition, comprising the steps of: mixing a pharmaceutically acceptable carrier with the compound of general formula (I) or its crystal form, pharmaceutically acceptable salt, hydrate or The solvates are mixed to form the pharmaceutical composition.
[0224] The present invention also provides a method of treatment, which comprises the steps of: administering the compound of general formula (I), or a crystalline form, pharmaceutically acceptable salt, hydrate or solvate thereof, as described in the present invention to a subject in need of treatment , or administer the pharmaceutical composition of the present invention for selectively inhibiting SOS1.
[0225] Compared with the prior art, the present invention has the following main advantages:
[0226] (1) The compound has a good selective inhibitory effect on SOS1;
[0227] (2) The compound has better in vitro and in vivo pharmacodynami...
Embodiment 1
[0237] Example 1(R)-N-(1-(3-(3-amino-5-(trifluoromethyl)phenyl)ethyl)-2-methyl-7,8-dihydro-[1, 4] Preparation of dioxin[2,3-g]quinazolin-4-amine
[0238]
[0239] The first step: preparation of 2,3-dihydrobenzo[b][1,4]dioxin-6-carbonitrile
[0240] 3,4-Dihydroxybenzonitrile (5g, 37mmol) was added to N,N-dimethylformamide (50mL), followed by 1,2-dibromoethane (28g, 148mmol) and potassium carbonate (26g, 185 mmol). The obtained reaction solution was stirred at 80 degrees overnight, then cooled down, poured into 500 mL of water, and extracted three times with 100 mL of ethyl acetate. The combined organic phases were dried and filtered, the filtrate was concentrated, and the obtained residue was separated by silica gel column chromatography (PE:EA=5:1) to obtain the target product (3.5 g, yield: 58%).
[0241] LC-MS: m / z 162 (M+H) + . 1 H NMR (400MHz, CDCl 3 )δ7.15-7.13(m,2H),6.92-6.90(m,1H),4.33-4.31(m,2H),4.29-4.27(m,2H).
[0242] Step 2: Preparation of 7-nitro-2,3-dih...
Embodiment 2
[0258] Example 2 N-((R)-1-(3-amino-5-(trifluoromethyl)phenyl)ethyl)-7-(methoxymethyl)-2-methyl-7,8 -Dihydro-[1,4]dioxo[2,3-g]quinazolin-4-amine and N-((R)-1-(3-amino-5-(trifluoromethyl) Phenyl)ethyl)-8-(methoxymethyl)-2-methyl-7,8-dihydro-[1,4]dioxo[2,3-g]quinazoline-4 - Preparation of amine mixtures
[0259]
[0260] LC-MS: m / z 449 (M+H) + . 1 H NMR (400MHz, DMSO-d 6 )δ7.98-7.91(m,2H),6.99(s,1H),6.88(s,1H),6.83(s,1H),6.68(s,1H),5.52-5.47(m,3H),4.49 -4.40(m, 2H), 4.15-4.09(m, 1H), 3.64-3.62(m, 2H), 3.34-3.33(m, 3H), 2.33(s, 3H), 1.52-1.50(m, 3H) .
[0261] Example 2 Four isomers were obtained by chiral separation
[0262]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com