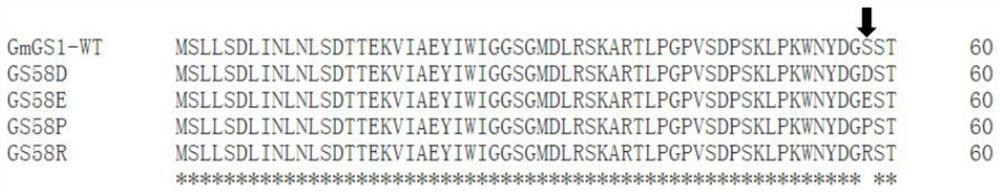Method for obtaining protein with glufosinate-ammonium resistance and mutant thereof
A glufosinate-ammonium and mutant technology, applied in the field of genetic engineering, can solve problems affecting plant growth and development, disturbing plant nitrogen metabolism, consumer resistance, etc., and achieve the effect of satisfying growth and development and normal nitrogen metabolism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0120] The rice (Oryza sativa) glutamine synthase (GS1) mutant provided in this example is obtained from the wild-type rice glutamine synthase itself (named OsGS1-WT, and the amino acid sequence is shown in SEQ ID NO.1, The 58th amino acid residue S of the coding nucleotide sequence is SEQ ID NO.5) is mutated to A, C, D, E, G, H, I, K, L, M, P, Q, R, T, Y or deletion (X) was obtained, and the obtained rice GS1 mutants were named OS58A, OS58C, OS58D, OS58E, OS58G, OS58H, OS58I, OS58K, OS58L, OS58M, OS58P, OS58Q, OS58R, OS58T, OS58Y, OS58X, respectively.
[0121] The amino acid sequence alignment of OS58A, OS58C, OS58D, OS58E, OS58G, OS58H, OS58I, OS58K, OS58L, OS58M, OS58P, OS58Q, OS58R, OS58T, OS58Y, OS58X and wild-type rice GS1 is as follows figure 2 As shown, in the figure: the position indicated by the arrow is the mutation site.
[0122] In this example, the coding sequence of each rice GS1 mutant is at the position encoding the 58th amino acid, and the codons used for t...
Embodiment 2
[0127] The soybean (Glycine max) GS1 mutant provided in this example is composed of wild-type soybean GS1 itself (named as GmGS1-WT, the amino acid sequence is shown in SEQ ID NO. 3, and the coding nucleotide sequence is SEQ ID NO. 7) The 58th position (corresponding to the 58th position of the reference sequence (SEQ ID NO. 1)) is obtained by mutating the amino acid residue S to D, E, P, and R. The obtained soybean GS1 mutants were named GS58D, GS58E, GS58P, GS58R.
[0128] The amino acid sequence alignment of soybean GS1 mutants GS58D, GS58E, GS58P, GS58R and wild-type soybean GS1 is as follows image 3 As shown, in the figure: the position indicated by the arrow is the mutation site.
[0129] In this example, the coding sequence of each soybean GS1 mutant is at the position encoding the 58th amino acid, the codons used for the corresponding amino acid are shown in the table below, and the nucleotides in the remaining positions are the same as the corresponding wild-type co...
Embodiment 3
[0133] The maize (Zea mays) GS1 mutant provided in this example is composed of wild-type maize GS1 itself (named ZmGS1-WT, the amino acid sequence is shown in SEQ ID NO.2, and the coding nucleotide sequence is SEQ ID NO.6 ) at position 58 (corresponding to position 58 of the reference sequence (SEQ ID NO. 1)) is obtained by mutating the amino acid residue S to D, E, I, K, P, R or deletion (X). The obtained maize GS1 mutants were named ZS58D, ZS58E, ZS58I, ZS58K, ZS58P, ZS58R, ZS58X, respectively.
[0134] The amino acid sequence alignment of maize GS1 mutants ZS58D, ZS58E, ZS58I, ZS58K, ZS58P, ZS58R, ZS58X and wild-type maize GS1 is as follows Figure 4 As shown, in the figure: the position indicated by the arrow is the mutation site.
[0135] In this example, the coding sequence of each maize GS1 mutant is at the position encoding the 58th amino acid, the codons used for the corresponding amino acid are shown in the following table, and the nucleotides in the remaining posit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com