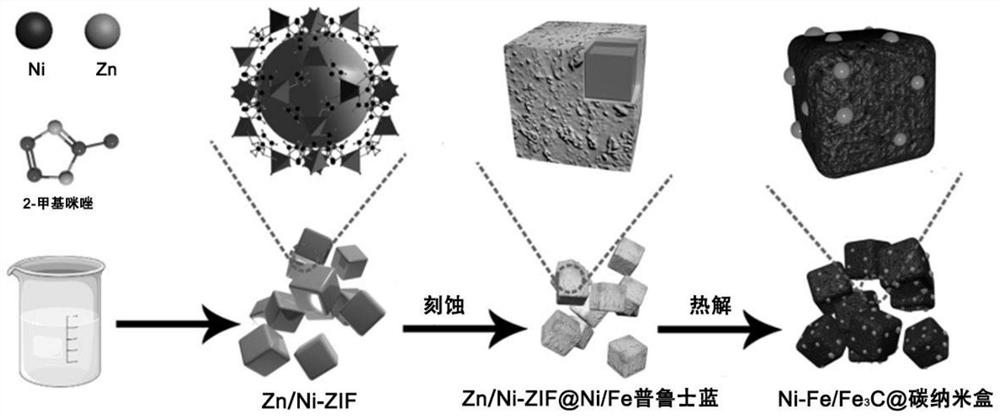
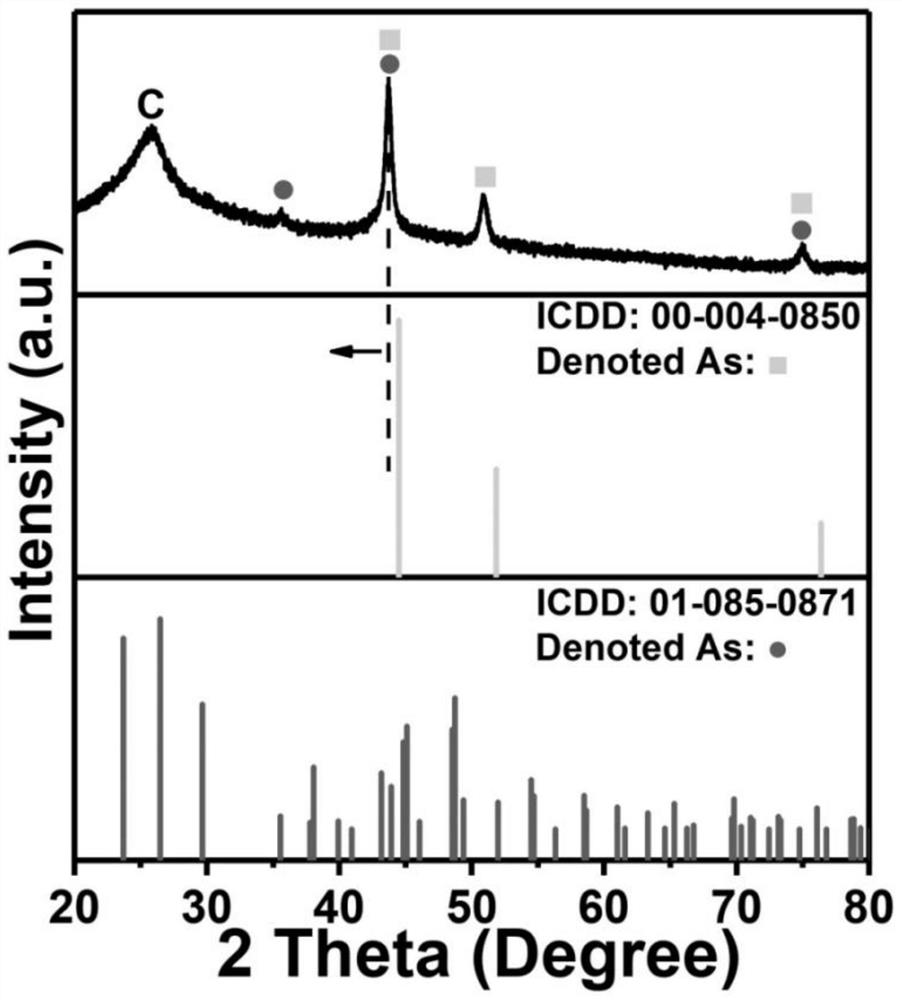
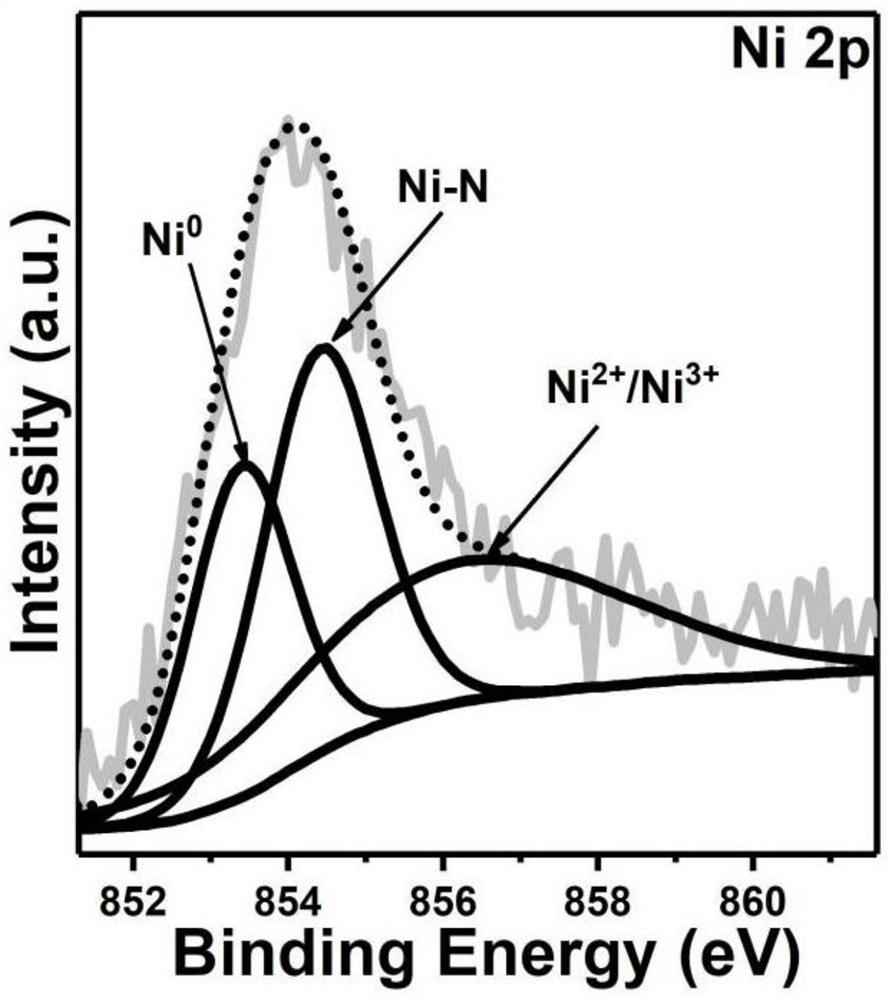
Iron-nickel-based or iron-cobalt-based Mott-Schottky electrocatalyst as well as preparation method and application thereof
An electrocatalyst, iron-nickel technology, applied in the field of iron-nickel-based or iron-cobalt-based Mott-Schottky electrocatalysts and preparations, can solve the problems of inability to fully expose active sites and unfavorable catalytic reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] The present embodiment provides a method for preparing an iron-nickel-based Mott-Schottky electrocatalyst, which specifically includes the following steps:
[0055] Preparation of S1, Zn-Ni-ZIF nanocubes
[0056] Dissolve 9.08g of 2-methylimidazole in 140mL of deionized water and stir to form solution A; mix 0.0634g of nickel nitrate hexahydrate, 0.6491g of zinc nitrate hexahydrate and 0.01g of cetyltrimethylammonium bromide (CTAB) was dissolved in 20 mL of deionized water to uniformly form solution B;
[0057] Add solution B to solution A while stirring, and after continuing to stir for a period of time, the solution is allowed to stand in a water bath at 40°C. The resulting precipitate is collected by a centrifuge and washed with deionized water and absolute ethanol. , and then dried to obtain Zn-Ni-ZIF nanocubes;
[0058] Preparation of S2, Zn-Ni-ZIF@Prussian Blue Analog Nanocubes
[0059] Disperse the Zn-Ni-ZIF nanocubes in deionized water at a concentration of 0...
Embodiment 2
[0074] A preparation method of an iron-cobalt-based Mott-Schottky electrocatalyst, comprising the following steps:
[0075] Preparation of S1, Zn-Co-ZIF nanocubes
[0076] Dissolve 2-methylimidazole in deionized water with a concentration of 0.0649 g / mL, stir to form solution A2; dissolve cobalt nitrate hexahydrate, zinc nitrate hexahydrate and CTAB in deionized water, and stir to form solution B2, wherein The concentration of cobalt nitrate hexahydrate in solution B2 is 0.00317g / mL, the concentration of zinc nitrate hexahydrate is 0.0325g / mL, and the concentration of CTAB is 0.0005g / mL;
[0077] Mix solution A2 and solution B2 in a volume ratio of 1:1 under stirring, stir while mixing, stand still in a water bath at 30-60 °C, collect the precipitate by centrifugation, use deionized water and anhydrous Washed with ethanol and dried to obtain Zn-Co-ZIF nanocubes;
[0078] Preparation of S2, Zn-Co-ZIF@Prussian Blue Analog Nanocubes
[0079] Disperse Zn-Co-ZIF nanocubes in dei...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| half wave potential | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com