Pharmaceutical composition, itraconazole pellet, preparation method and itraconazole capsule
A technology of itraconazole pellets and itraconazole is applied in the field of itraconazole capsules and pharmaceutical compositions to achieve the effects of reducing the incidence or severity, reducing the amount of use and promoting absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
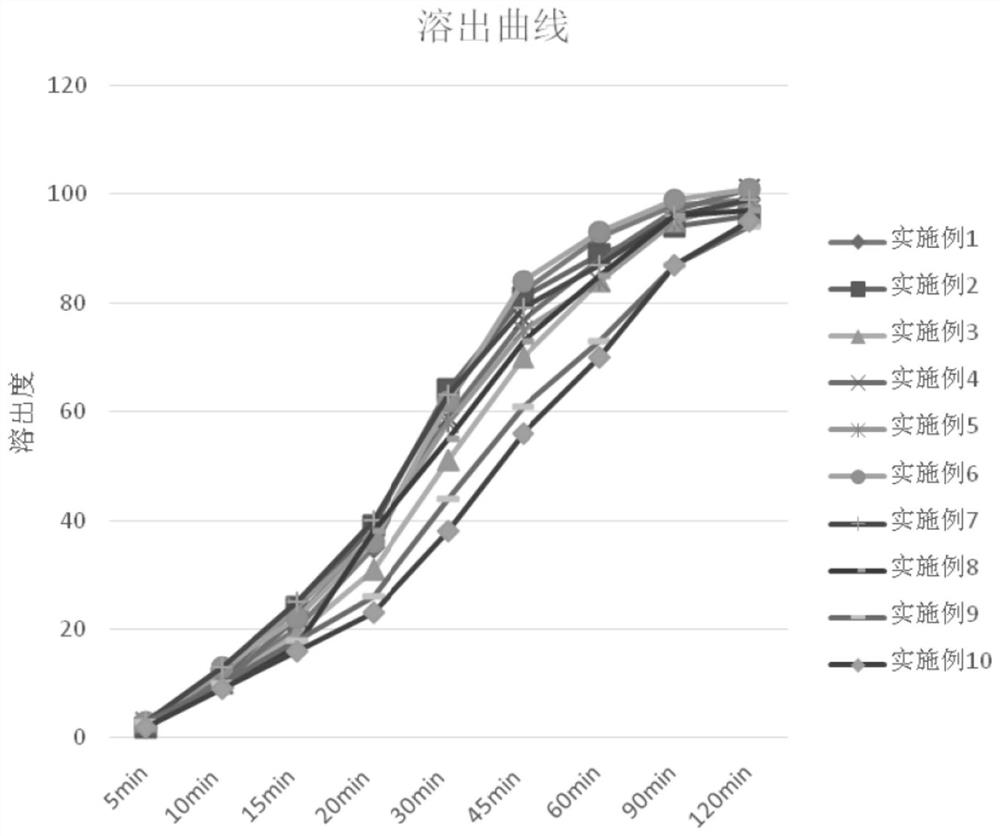
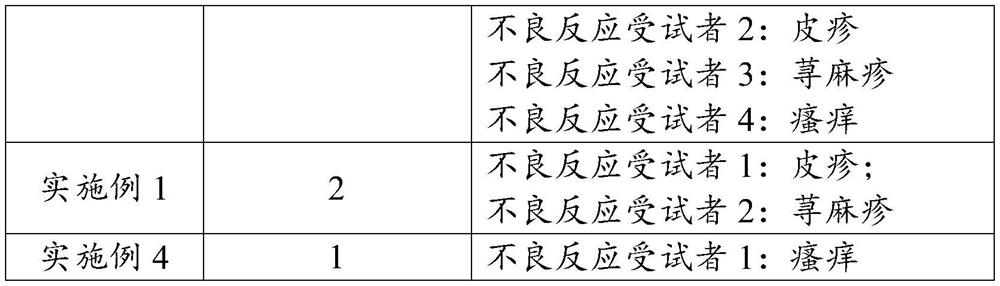
Image
Examples
Embodiment 1
[0054] The present embodiment provides a preparation method of itraconazole pellets, comprising the following operations:
[0055] (1) Drug-containing layer coating:
[0056] A. Take 20g of sodium lauryl sulfate (lubricant), dissolve in 1000g of 95% ethanol, add 200g of hydroxypropyl methylcellulose 5cps (second binder), and disperse evenly;
[0057] B, get itraconazole 75g, rhubarb aconite extract (penetration enhancer) 6g and be dispersed in the solution obtained in item A, then add 1500g methylene chloride, stir and disperse evenly, wherein the raw rhubarb and The dosage ratio of Cannon Fuzi is 1:1;
[0058] C, get talcum powder (lubricant) 15g and be dispersed in the 95% ethanol of 200g;
[0059] D, the solution obtained in item C is added to the solution obtained in item B after passing through a 325 mesh screen, and stirred to obtain a drug-containing suspension;
[0060] E, get starch sucrose ball core (blank ball core) 100g and put it in the coating pot, heat, after...
Embodiment 2
[0069] The present embodiment provides a preparation method of itraconazole pellets, comprising the following operations:
[0070] (1) Preparation of pill-containing core:
[0071] Dissolve 6 g of rhubarb aconite extract (penetration enhancer) and 54 g of sucrose (first binder) in water, add 50 g of starch (filler), and then granulate through a fluidized bed to obtain a 20-25 mesh containing The pill core, wherein the dosage ratio of raw rhubarb and aconite in the preparation of rhubarb aconite extract is 1:1.
[0072] (2) Drug-containing layer coating:
[0073] A. Take 20g of sodium lauryl sulfate (lubricant), dissolve in 1000g of 95% ethanol, add 200g of hydroxypropyl methylcellulose 5cps (second binder), and disperse evenly;
[0074] B, get itraconazole 75g and be dispersed in the obtained solution of item A, then add 1500g methylene chloride, stir and disperse uniformly;
[0075] C, get talcum powder (lubricant) 15g and be dispersed in the 95% ethanol of 200g;
[0076]...
Embodiment 3
[0086] The present embodiment provides a preparation method of itraconazole pellets, comprising the following operations:
[0087] (1) Drug-containing layer coating:
[0088] A. Take 20g of sodium lauryl sulfate (lubricant), dissolve in 1000g of 95% ethanol, add 200g of hydroxypropyl methylcellulose 5cps (second binder), and disperse evenly;
[0089] B, get itraconazole 75g, rhubarb Aconite Asarum extract (penetration enhancer) 6g and disperse in the solution obtained in item A, then add 1500g of dichloromethane, stir and disperse evenly, use when preparing Rhubarb Aconite Asarum extract The dosage ratio of raw rhubarb, aconite and asarum is 1:1:0.6;
[0090] C, get talcum powder (lubricant) 15g and be dispersed in the 95% ethanol of 200g;
[0091] D, the solution obtained in item C is added to the solution obtained in item B after passing through a 325 mesh screen, and stirred to obtain a drug-containing suspension;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com