Hmo mixtures
A composition, a technology of symptoms, applied in the field of HMO mixtures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
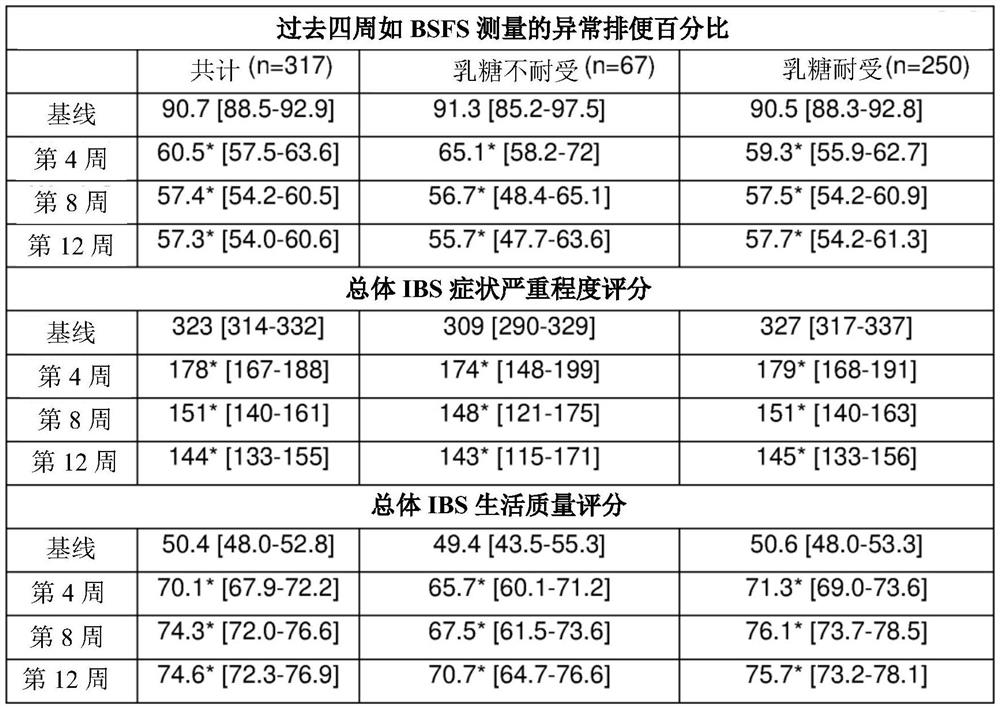
[0090] A total of 317 male and female patients from 17 clinics were recruited for the study. Patients were eligible to participate in the study if they were: over 18 years of age at screening; had a current clinical diagnosis of IBS from a healthcare provider; and met Rome IV diagnostic criteria for IBS. Patients will be excluded from participation if they have been diagnosed with celiac disease, diverticulitis, inflammatory bowel disease or Clostridium difficile infection.
[0091] All patients underwent screening examinations at clinics where written informed consent was obtained, and inclusion and exclusion criteria were checked. After enrollment in the trial, patients filled out an electronic basic survey (Survey 1). The survey includes a number of validity questionnaires such as the Bristol Stool Type Scale (BSFS), IBS Symptom Severity Score (IBS-SSS), IBS-specific Gastrointestinal Symptom Rating Scale (GSRS-IBS) and IBS Quality of Life ( IBS-QoL). Patients also indica...
Embodiment 2
[0101] The ability of different HMOs to increase Bifidobacterium abundance in IBS feces was evaluated in a batch culture fermentation system. The following HMOs were tested: 2'-FL, LNnT, LNT, 3'-SL, 6'-SL and a mixture of 2'-FL and DFL.
[0102] Fecal samples were collected from IBS-D patients, IBS-C patients and healthy volunteers. Batch fermenters (working volume 300 ml) were set up and each was aseptically inoculated with a fecal sample, basal medium and one HMO. The temperature was maintained at 37°C (human body temperature) by a circulating water bath, and the pH was adjusted to 6.8.
[0103] The batch fermenter was run for 48 hours with samples taken at 0 hours, 8 hours and 48 hours. Samples were analyzed using:
[0104] • Use fluorescence in situ hybridization (FISH) in combination with flow cytometry (Flow-FISH) to enumerate bacterial populations. Eleven genotypic probes were used to target specific regions of 16S rRNA for major bacterial taxa of the gut microbiota...
Embodiment 3
[0107] HMO 2'-FL and LNnT were introduced into the rotary mixer at a mass ratio of 4:1. 0.25w% magnesium stearate was added to the mixer and the mixture was mixed for 10 minutes. The mixture was then coagulated in a fluid bed, filled into 5 gram stick packs, and sealed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com