CAR-T cells targeting IL-1RAP and their use in acute myelogenous leukemia (AML)
An acute myeloid, leukemia technology, applied in the field of IL-1RAP-targeting CAR-T cells and their use in acute myeloid leukemia (AML), can solve the lack of efficacy, no second remission, inability to Tolerance procedures, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0141] Example 1: Monoclonal Antibody Production
[0142] Mouse anti-hIL-1RAP monoclonal antibodies were generated by standard hybridoma technology.
[0143]Briefly, BALB / c mice (5 weeks, Charles River) were treated with a compound consisting of the extracellular part of IL-1 RAP (NM_002182.2, NCBI) and the Fc part of human IgG1 (R&D Systems, Lille, France). Recombinant fusion proteins were immunized by footpad (n=3) or intraperitoneally (n=5). Lymph node or splenocytes as well as blood samples were harvested and cells were fused with mouse myeloma cell lines and then analyzed by FACS (Becton Dickinson) against IL-1RAP positive cell line (KU812) and IL-1RAP negative cell line (Raji, KG1) ) to filter.
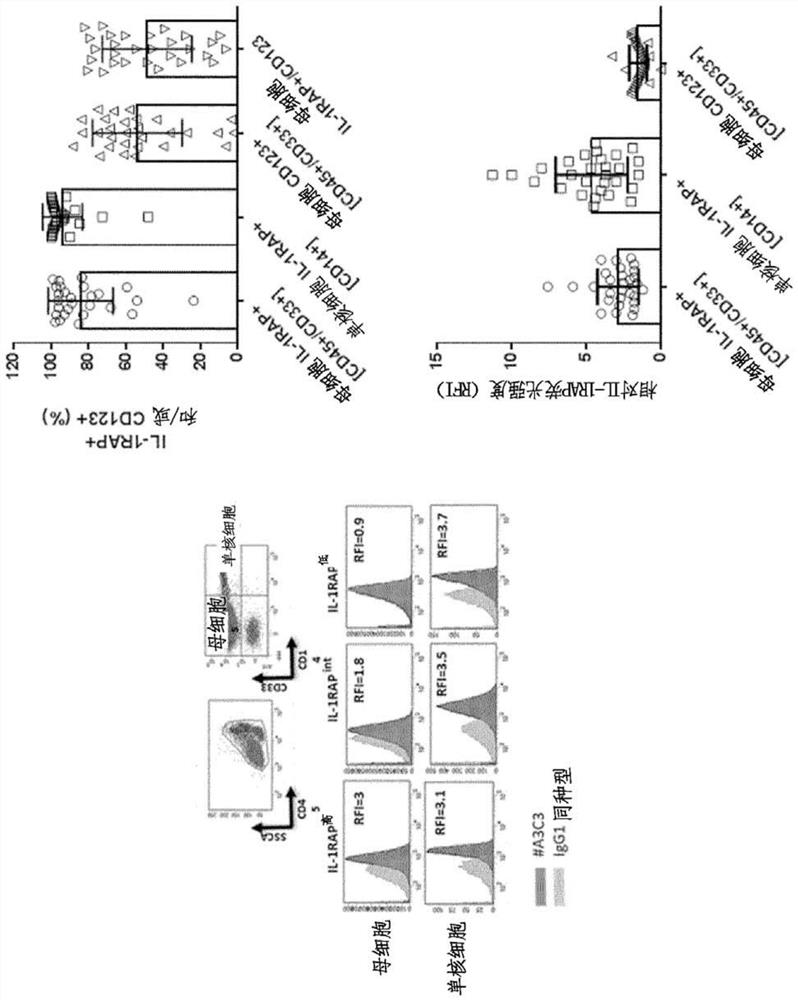
[0144] Screening of hybridomas enables selection of IL-1 RAP-positive cell lines (KU812 or KG-1, AML or Phi, respectively) + p 210 CML) and negative cell lines (Tom-1, NALM-20, Jurkat or Raji, respectively Phi+ p190 B-ALL, Phi - B-ALL, T-ALL or Burkitt's lymphoma) 5 mono...
Embodiment 2
[0166] Example 2: Lentiviral Constructs
[0167] Based on molecular sequencing of VDJ or VJ rearrangements and CDR3 nucleotide sequence determination, single-chain variable fragments (scFv) generated by synthesis from the #E3C3 IL-1RAP hybridoma of Example 1 were cloned into the SIN-pSDY backbone Zhonglai prepared CAR lentiviral construct (pSDY-iC9-IL-1RAPCAR-dCD19) (Rossolillo P, Winter F, Simon-Loriere E, Gallois-Montbrun S, Negroni M. Retrovolution: HIV-driven evolution of cellulargenes and improvement of anticancer drug activation. PLoS Genet. 2012;8(8):e1002904).
[0168] Briefly, a SIN lentiviral construct was constructed carrying the iCASP9 safety cassette, the single chain variable fragment of the #E3C3 monoclonal antibody, and the cell surface expressed marker ΔCD19 for monitoring and potential cell selection. All three of these transgenes were separated by a 2A peptide cleavage sequence and were under the control of the EF1 promoter and SP163 enhancer sequence (part...
Embodiment 3
[0170] Example 3: Generation of IL-1RAP CART cells
[0171] CD3+ T lymphocytes obtained from peripheral blood mononuclear cells of healthy donors were activated with anti-CD3 / CD28 beads (Life Technologies, France) according to the manufacturer's instructions, and then incubated on magnetic columns (MACS, Miltenyi Biotec, Paris, France) ) to separate. On day 2, activated T cells were transduced by spinoculation using the lentiviral vector of Example 2 at 2000 g for 90 min at 10°C in contact with the supernatant (SN). Transduction efficiency was determined by performing flow cytometry analysis to identify ΔCD19 cell surface marker expression. Four days after transduction, CD19 positive cells labeled with CD19 microbeads (Miltenyi Biotec, Paris, France) were magnetically separated using a MACS column. Isolated CD19 expressing cells were expanded in complete X-vivo medium (Lonza, Bale, Switzerland) containing 500 UI / mL rhIL-2 (Proleukin; Novartis) supplemented with 8% human seru...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com