Photoactivated porphyrin prodrug ternary assembly as well as preparation method and application thereof
A technology of assembly and photoactivation, which is applied in the direction of pharmaceutical formulations, drug combinations, preparations for in vivo experiments, etc., can solve the problems of lack of targeting, damage to the human immune system, poor water solubility, etc., and achieve weakening of π-π accumulation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] The application also provides a preparation method of the photoactivated porphyrin prodrug ternary assembly, including:
[0037] The aqueous solution of the porphyrin prodrug and the aqueous solution of the permethylated β-cyclodextrin-modified hyaluronic acid are mixed and sonicated to obtain a photoactivated porphyrin prodrug ternary assembly.
[0038]In the above process, the antitumor drug molecule modified by the carboxyphenyl porphyrin and the photooxidative active functional group is also called a porphyrin prodrug (TPP-CA4), and its preparation method is specifically:
[0039] The antitumor drug molecule modified by aminoacrylate bond is esterified with monocarboxyphenyl porphyrin under the action of a catalyst to obtain the antitumor drug molecule modified with carboxyphenyl porphyrin and photooxidative active functional group.
[0040] In the above process, the aminoacrylate bond-modified antitumor drug Combretastatin A-4 is synthesized according to the litera...
Embodiment 1
[0048] Embodiment 1 A preparation method of a light-activated permethylated-β-cyclodextrin-modified hyaluronic acid-porphyrin prodrug ternary supramolecular assembly based on in-situ drug release and photodynamic therapy, the steps are as follows :
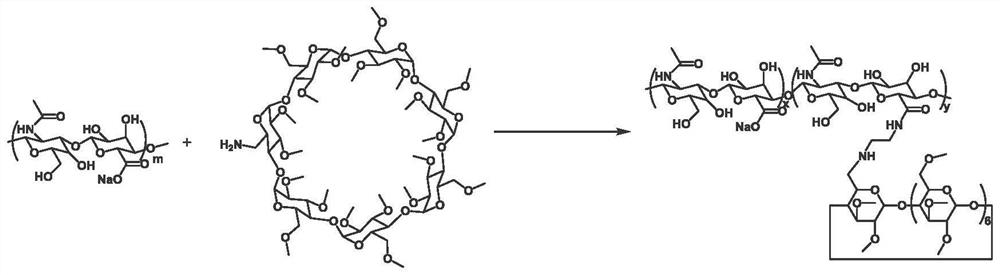
[0049] 1) Synthesis of permethylated β-cyclodextrin-modified hyaluronic acid (HA-PMeCD)
[0050] 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) (0.875 mmol, 167.7 mg), N-hydroxysuccinimide sulfonic acid sodium salt (NHSS) ( 0.875 mmol, 190 mg) was added to 100 mg of sodium hyaluronate (Mw=93,000) dissolved in 30 mL of phosphate buffer (PBS, 0.1 M, pH=7.2), and stirred at room temperature for 0.5 hours. Subsequently, 120 mg of mono-6-deoxy-6-amino-permethylated-β-cyclodextrin dissolved in 10 mL of PBS solution was added to the reaction solution, and stirring was continued at room temperature for 24 hours. After the reaction was completed, dialysis was performed with deionized water for 5 days, and the HA-PMeCD ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com