Refining method of iopamidol intermediate
A technology of iopamidol and asana, which is applied in the field of refining iopamidol intermediates, can solve the problems that iopamidol is difficult to reach the pharmaceutical grade, increase the difficulty of iopamidol synthesis route, and unfavorable reactions, so as to simplify production Process, easy operation, thorough reaction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1, the preparation of compound of formula V
[0055] In 3L reaction kettle, add 450g formula II compound (this compound can be prepared according to the method in CN103086915A patent) and 2L purified water, be warming up to 70-80 ℃ of dissolving, transfer in 3L hydrogenation kettle, add 9g 5% palladium carbon , the temperature is controlled at 60-70°C, and the pressure is maintained at 0.6-1.4Mpa to carry out the hydrogenation reaction. After the reaction is completed for 5-10h, the filtrate is obtained by filtering.
Embodiment 2
[0056] Embodiment 2, the preparation of iopamidol intermediate formula III compound
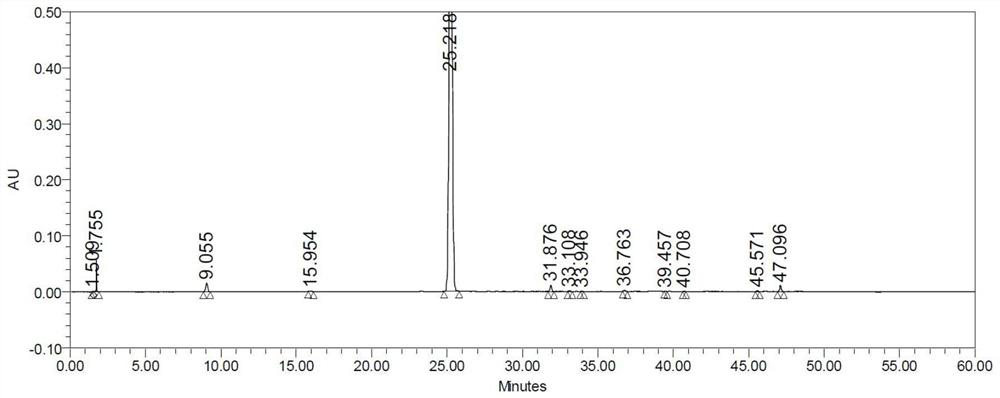
[0057] In the reactor of 5L, add 500g water, sodium chloride 243g, iodine 374g, potassium iodate 158g, CF 3 CO 2 Ag14g, cooled to 5-20°C, and 456g of refined hydrochloric acid was added dropwise. After the addition, the temperature was controlled to 50-60°C and the filtrate obtained in Example 1 was slowly added dropwise, and the reaction was incubated for 17h. The temperature was controlled below 60°C, 50% sodium hydroxide solution was added dropwise, the pH was adjusted to 5-6, the temperature was slowly lowered to 10-25°C, the stirring was continued for 3 hours and then filtered, the filter cake was washed with 800g of water, and dried under reduced pressure to obtain 888g of intermediate formula III Compound, yield 99.9%, purity 96.7%, no diiodo-substituted impurity 1 compound was detected. The HPLC chromatogram of the diiodo-substituted impurity 1 compound is shown in the attached fi...
Embodiment 3
[0060] Embodiment 3, the preparation of iopamidol intermediate formula III compound
[0061] In the reactor of 5L, add 500g water, sodium chloride 243g, iodine 374g, potassium iodate 158g, CF 3 CO 2 Ag5.6g, cooled to 5-20°C, and 456g of refined hydrochloric acid was added dropwise. After the addition, the temperature was controlled at 50-60°C and the filtrate obtained in Example 1 was slowly added dropwise, and the reaction was incubated for 17h. The temperature is controlled below 60°C, 50% sodium hydroxide solution is added dropwise, the pH is adjusted to 5-6, the temperature is slowly lowered to 10-25°C, the stirring is continued for 3 hours and then filtered, the filter cake is washed with 800g of water, and dried under reduced pressure to obtain 826g of intermediate formula III Compound, yield 93%, purity 96.1%, no diiodine substituted impurity 1 compound was detected. The HPLC detection spectrum of the compound of formula III prepared in this example is as attached i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com