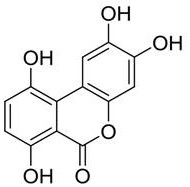
High-purity gentisic acid and application thereof
A gentisic acid, high-purity technology, applied to high-purity gentisic acid and its application field, can solve the problems of poor product purity and low yield of gentisic acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0167] Feeding reaction: add 5.0mol / L NaOH solution to the reaction kettle, add 50g of 5-bromosalicylic acid, 0.5g of cuprous iodide, and 0.5g of oxalic acid; under nitrogen protection, start stirring; heat up to 100~120℃ for 24h, Sampling control HPLC (5-bromosalicylic acid)≤1.5%; cool down to 20~30℃, add water;
[0168] Acidification: control the temperature to 20~30℃, add concentrated hydrochloric acid to adjust the pH of the reaction solution to (2.4), there is solid precipitation, filter;
[0169] Extraction: add 50 g of sodium chloride and 250 g of methyl tert-butyl ether to the filtrate, stir and extract, and flocs appear after standing;
[0170] Filtration: 5 g of diatomaceous earth was added to the Buchner funnel, filtered and separated to obtain the first organic phase and the aqueous phase; 125 g of methyl tert-butyl ether was added to the aqueous phase, filtered and separated to obtain the second organic phase. 2 and organic phase 1 are combined to obtain organic ...
Embodiment 2
[0177] Feeding reaction: add 5.0mol / L NaOH solution to the reaction kettle, add 200g of 5-bromosalicylic acid, 1.8g of cuprous chloride, and 2g of oxalic acid; under nitrogen protection, turn on stirring; heat up to 100~120℃ for 24h reaction, take samples In-control HPLC (5-bromosalicylic acid)≤1.5%; cool down to room temperature (20~30℃), add water;
[0178] Acidification: control the temperature to 20~30℃, add concentrated hydrochloric acid to adjust the pH of the reaction solution to 2.4, there is solid precipitation, and filter;
[0179] Extraction: add 200 g of sodium chloride and 1000 g of methyl tert-butyl ether to the filtrate, stir and extract, and flocs appear after standing;
[0180] Filtration: add 50 g of diatomaceous earth to the Buchner funnel, filter, and separate the liquids to obtain an organic phase 1 and an aqueous phase; add 500 g of methyl tert-butyl ether to the aqueous phase, filter, and separate liquids to obtain an organic phase 2. 2 and organic phase ...
Embodiment 3
[0187] Feeding reaction: add 5.0mol / L KOH solution to the reaction kettle, add 500 g of 5-bromosalicylic acid, 5.0 g of copper sulfate pentahydrate, and 5 g of oxalic acid; under nitrogen protection, start stirring; heat up to 100~120 ° C for 24 hours, and take samples In-control HPLC (5-bromosalicylic acid)≤1.5%; cool down to room temperature (20~30℃), add water;
[0188] Acidification: control the temperature to 20~30℃, add concentrated hydrochloric acid to adjust the pH of the reaction solution to 2.4, there is solid precipitation, and filter;
[0189] Extraction: Add 500 g of sodium chloride and 250 g of methyl tert-butyl ether to the filtrate, stir and extract, and flocs appear after standing;
[0190] Filtration: add 50 g of diatomaceous earth to the Buchner funnel, filter, and separate the liquids to obtain an organic phase 1 and an aqueous phase; add 125 g of methyl tert-butyl ether to the aqueous phase, filter, and separate liquids to obtain an organic phase 2. 2 and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com