Probe composition, reagent, kit and detection method for detecting mucopolysaccharide storage disease type VII
A technology for the storage and composition of mucopolysaccharides, which is applied in biochemical equipment and methods, recombinant DNA technology, and the determination/testing of microorganisms, to achieve the effects of reducing birth rate, improving diagnostic accuracy, and improving detection timeliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] The specific method of using the above kit:
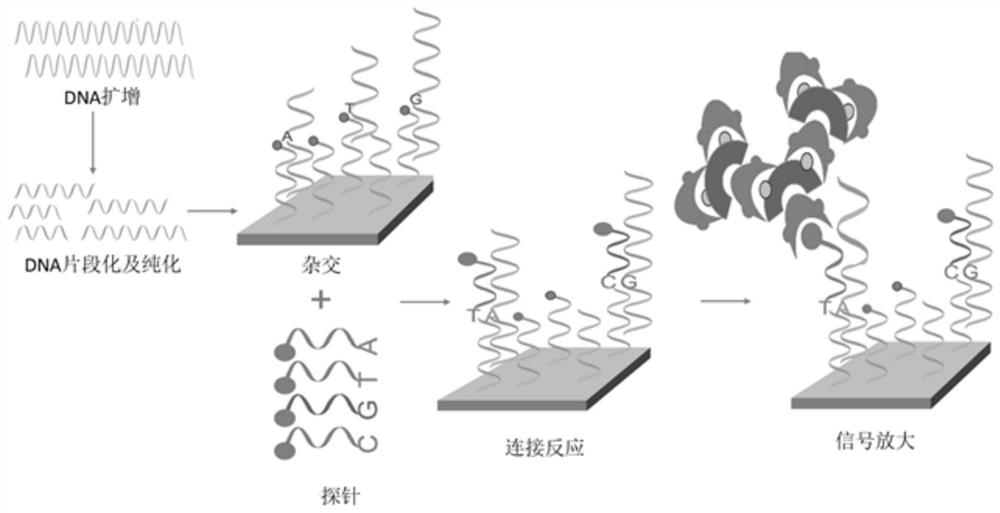
[0108] 1. DNA amplification
[0109] Configure Denaturation Master Mix according to the following system: (All reagents are independently developed and prepared from purchased raw materials, and are currently applying for a first-class registration certificate, tentatively named CNVPLUS TM Supporting detection reagents for chip scanners)
[0110] Form 2
[0111] Element 1× Denaturing solution (Denat Soln 10X) 2μl water 18μl
[0112] Configure the amplification master mix (Amplification Master Mix) according to the following system: Vortex to mix the components of the amplification master mix, then spin the tube twice, and vortex again to get the amplification master mix.
[0113] Form 3
[0114]
[0115] S1. Take 20 μl of DNA sample (5 ng / μL), add 20 μl of the above-mentioned denaturing mixture, and incubate at room temperature for 10 minutes.
[0116] S2. Add 130 μl neutral solution (Neu...
Embodiment 2
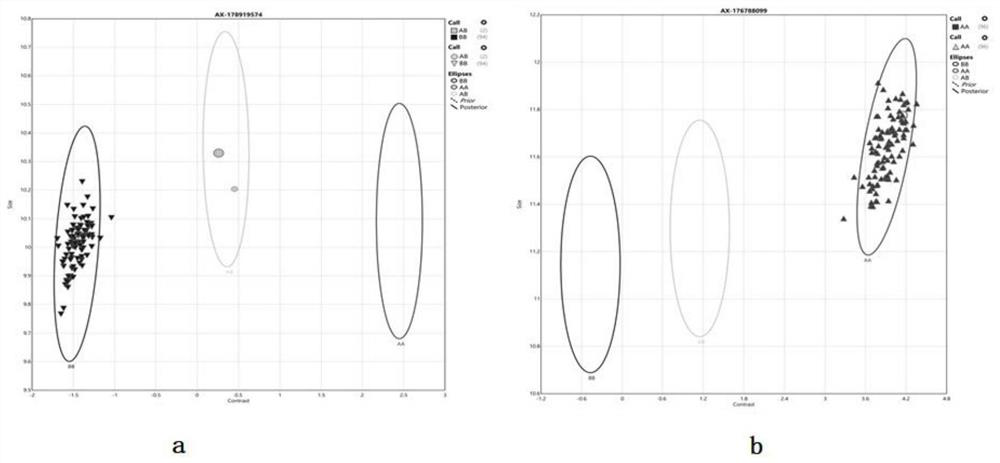
[0175] The above chips were used to verify GUSB positive sites
[0176] Step 1. Sample collection: after informed consent, peripheral blood was collected from 12 patients, including 7 male patients and 5 female patients, as verification samples.
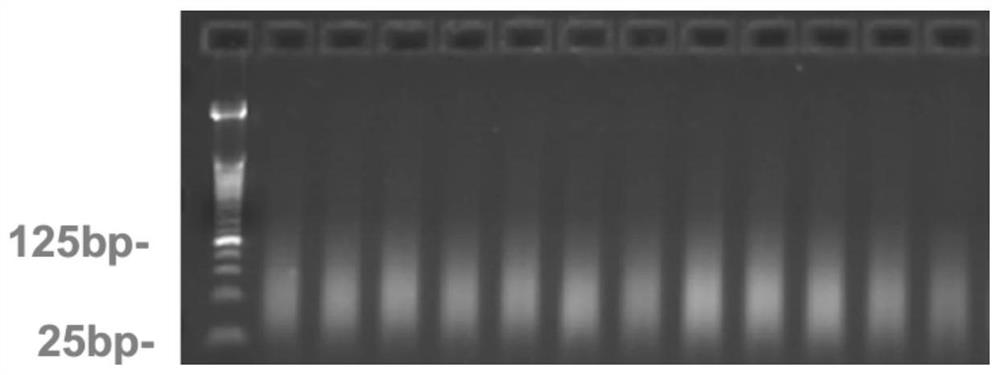
[0177] Step 2. Sample preparation: DNA extraction is performed on the above verification samples, and quality control (such as S14 quality detection) is performed by gel electrophoresis, Nanodrop spectrophotometer, etc., to ensure that each DNA sample has no degradation, no impurities, and high purity . Optical Density (OD) 260 / 280nm ratio ranges from 1.8 to 2.0, and OD 260 / 230nm ratio ranges from 1.5 to 2.0. Samples that do not meet any of the conditions need to be purified.
[0178] Step 3. Detection reaction: The samples that pass the quality inspection are first subjected to whole-genome amplification, and the amplified products are then subjected to enzyme digestion, precipitation recovery, and resuspension to obtain DNA fragme...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com