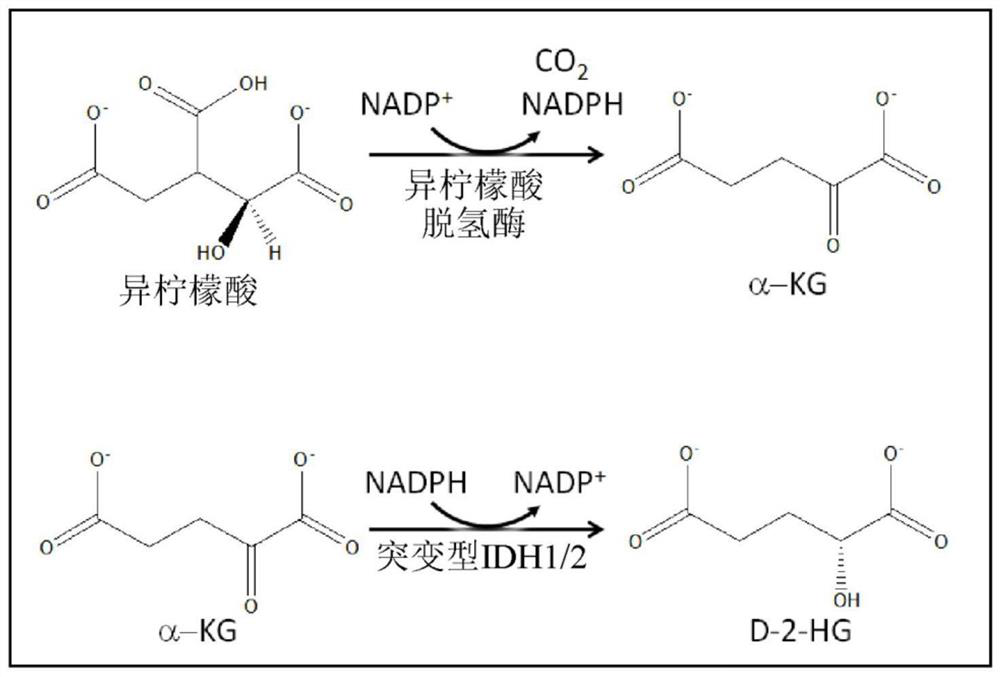
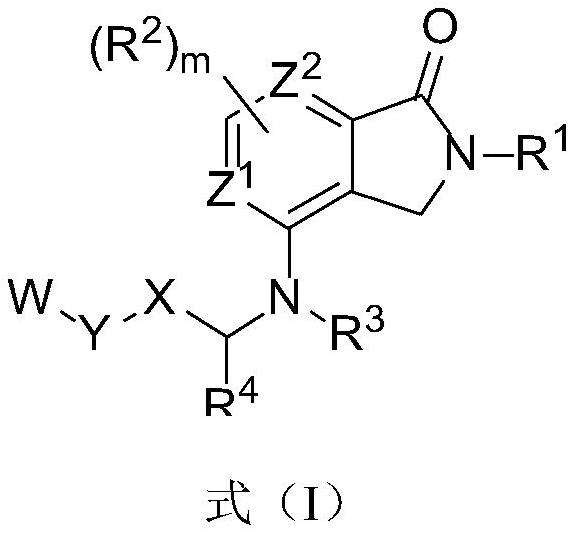
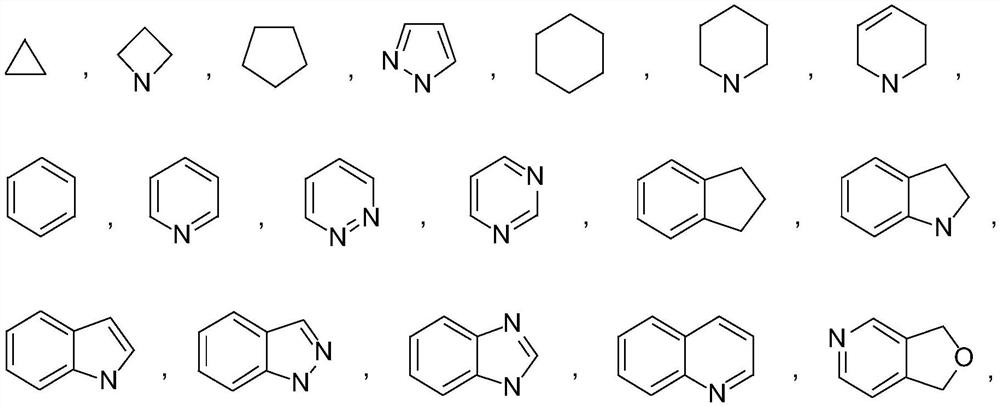
Isocitrate dehydrogenase (IDH) inhibitors
An unsaturated, free technology that can be used in the field of pharmaceuticals for diseases to solve problems such as damage to catalysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0201] The preparation of compounds of the present disclosure can involve the protection and deprotection of various chemical groups. The need for protection and deprotection and the selection of appropriate protecting groups can be readily determined by those skilled in the art. The chemistry of protecting groups can be found in, for example, T.W. Greene and P.G.M. Wuts, Protective Groups in Organic Synthesis, 3rd Edition, Wiley & Sons , Inc.), New York (1999), which is incorporated herein by reference in its entirety.
[0202] The reaction can be monitored according to any suitable method known in the art. For example, by spectroscopic means, such as nuclear magnetic resonance spectroscopy (eg 1 H or 13 C), infrared spectroscopy, spectrophotometry (eg UV-visible light), mass spectrometry, or monitoring of the product by chromatography, eg high performance liquid chromatography (HPLC), liquid chromatography-mass spectrometry (LCMS) or thin layer chromatography (TLC) form....
example
[0244] The general methods of the present disclosure are further elucidated below. The compounds of the present disclosure can be prepared by methods known in the art. The detailed preparation methods of the preferred compounds of the present disclosure are described below. However, these in no way limit the preparation methods of the compounds of the present disclosure.
Synthetic example
[0246] The structures of the compounds in the following examples were characterized by nuclear magnetic resonance (NMR) or / and mass spectrometry (ESI). NMR shifts (δ) are in 10 -6 (ppm) is given in units. 1 H-NMR spectra were recorded in dimethyl sulfoxide-d on a VarianMercury VX 400 spectrometer with tetramethylsilane (TMS) as internal standard 6 (DMSO-d 6 ) or CDCl 3 middle.
[0247] ESI-HRMS measurements were performed using an Agilent 1260-6230TOF LC-MS mass spectrometer.
[0248] High performance liquid chromatography (HPLC) measurements were performed on an Agilent 1200LC using a Phenomen C18 column (4.6 mm x 150 mm, 0.4 μm).
[0249] Thin-layer chromatography was performed using Yantai Huanghai HSGF254 silica gel plates. Silica gel plates for thin layer chromatography (TLC) are 0.15mm to 0.2mm. The silica plates used for separation and purification of the product by TLC were 0.4 mm to 0.5 mm.
[0250] The purified chromatography column used silica gel as a carri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com