Precious metal methanol-to-hydrogen catalyst as well as preparation method and application thereof
A technology for producing hydrogen from methanol and precious metals, which is applied in the direction of catalyst activation/preparation, metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, and can solve the problems of low dispersion, high and low CO concentration in hydrogen-rich products CO selectivity and other issues, to achieve high temperature stability, inhibit the formation of carbon deposits, reduce the effect of CO concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
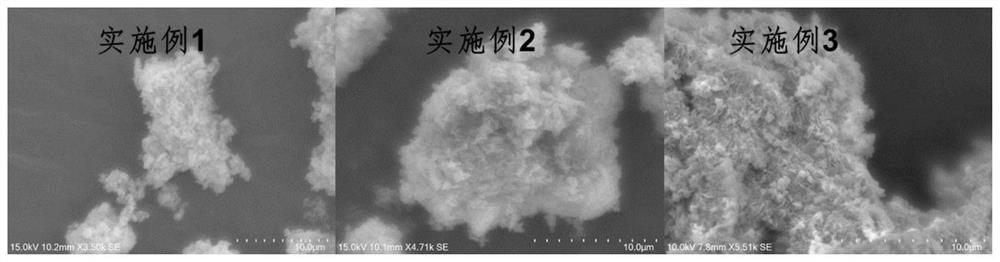
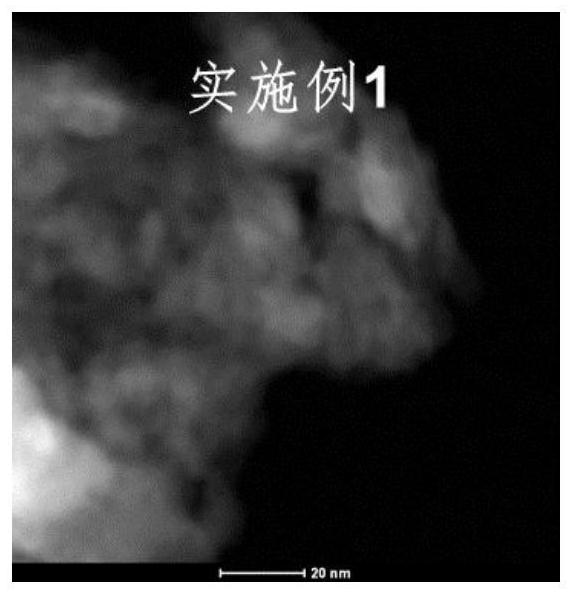
Embodiment 1
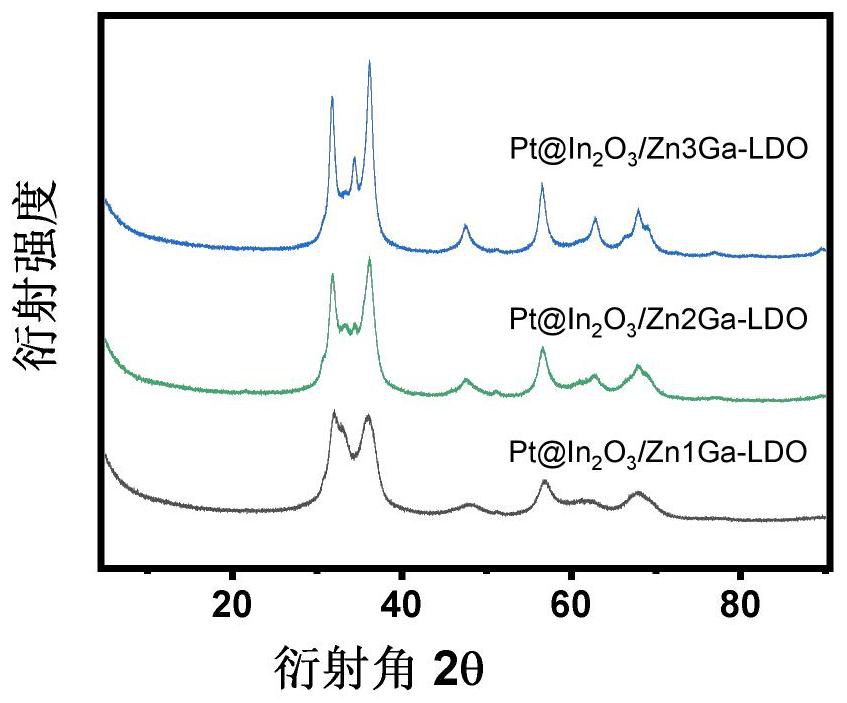
[0045] First, weigh 2.6gNa 2 CO 3 Dissolve in 50mL deionized water to prepare 0.5M Na 2 CO 3 solution; take the ratio of Zn to Ga as Zn:Ga=3:1 (mol:mol), weigh 11.2g of Zn(NO 3 ) 2 ·6H 2 O and 3.2 g of Ga nitrate were dissolved in 50 mL of deionized water to prepare 0.75 M of Zn 2+ solution and 0.25M Ga 3+ solution, gradually added dropwise to the aforementioned Na 2 CO 3 In the solution; 6.4g of NaOH was weighed and dissolved in 40mL of deionized water to prepare a 4M NaOH solution, and the pH value was kept at 10 by adding NaOH solution dropwise to the aforementioned metal mixed solution, and the hydrotalcite nanoparticles gradually precipitated; the crystallization process , stirred at room temperature for 17 h, filtered the suspension and washed the particles with deionized water until the pH was close to neutral, and then further washed with 400 mL of ethanol for 2 h, and then put the isolated solid matter in a vacuum drying box and dried for 8 h to obtain ZnGa-LD...
Embodiment example 2
[0049] Comparing implementation case 2 with implementation case 1, the difference is that the ratio of zinc to gallium is 2:1.
[0050] First, weigh 2.6gNa 2 CO 3 Dissolve in 50mL deionized water to prepare 0.5M Na 2 CO 3 solution; take the ratio of Zn to Ga as Zn:Ga=2:1 (mol:mol), weigh 10.4g of Zn(NO 3 )2·6H 2 O and 3.8 g of Ga nitrate were dissolved in 50 mL of deionized water to prepare 0.7 M of Zn 2+ solution and 0.3M Ga 3+ solution, gradually added dropwise to the aforementioned Na 2 CO 3 In the solution; 6.4g of NaOH was weighed and dissolved in 40mL of deionized water to prepare a 4M NaOH solution, and the pH value was maintained at 10 by adding dropwise NaOH solution to the aforementioned metal mixed solution, and the hydrotalcite nanoparticles gradually precipitated; After 17 h stirring at room temperature, the suspension was filtered and the particles were washed with deionized water until the pH was close to neutral, followed by further washing with 400 mL ...
Embodiment example 3
[0053] The implementation case 3 is compared with the implementation cases 1 and 2, the difference is that the ratio of zinc and gallium is 1:1.
[0054] First, weigh 2.6gNa 2 CO 3 Dissolve in 50mL deionized water to prepare 0.5M Na 2 CO 3 solution; take the ratio of Zn to Ga as Zn:Ga=1:1 (mol:mol), weigh 7.4g of hydrated zinc nitrate and 6.4g of hydrated gallium nitrate and dissolve it in 50mL of deionized water to prepare 0.5M of Zn 2+ solution and 0.5M Ga 3+ solution; Weigh 6.4g of NaOH and dissolve it in 40mL of deionized water to prepare a 4M NaOH solution. By adding NaOH solution dropwise to the aforementioned metal mixed solution, the pH value is kept at 10, and the hydrotalcite nanoparticles are gradually precipitated; then crystallized Procedure, stirring at room temperature for 17 h, the suspension was filtered and the particles were washed with deionized water to near neutral pH, followed by further washing with 400 mL of ethanol for 2 h. Put the separated soli...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com