Application of astragaloside in preparation of medicine for preventing Alzheimer's disease
A technology of Alzheimer's disease and astragaloside, which is applied in the field of biomedicine, can solve the problems that have not been fully studied, and achieve the effect of broadening the selection field, increasing the expression level, and inhibiting hypermethylation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] The preparation of relevant reagent in the embodiment is as follows:
[0025] 1. Preparation of Aβ oligomers
[0026] 1mg of Aβ 1-42 Powder (AnaSpec), fully dissolved with 222 μL pre-cooled hexafluoroisopropanol (HFIP, sigma) and shaken to mix well, let stand at room temperature for 60 minutes, and place on ice for 10 minutes to obtain 1mmoL / L Aβ 1-42 solution. Vacuum dry the volatile HFIP until clear Aβ is produced at the bottom of the EP tube 1-42 -HFIP-peptide film, long-term storage at -80°C. Take out the peptide film before use, add 44μL DMSO and mix well to make 5mM Aβ 1-42 mother liquor. Add 2156 μL of pre-cooled PBS to make 100 μmoL / L of Aβ 1-42 working fluid. Incubate at 4°C for 24h, centrifuge at 4°C / 14000x g for 10min, discard the precipitate and absorb the supernatant, filter through a filter, and obtain Aβ 1-42 Oligomers, aliquoted and stored at -80°C.
[0027] 2. Memantine configuration
[0028] Weigh 10 mg memantine hydrochloride (sigma) and dis...
Embodiment 1
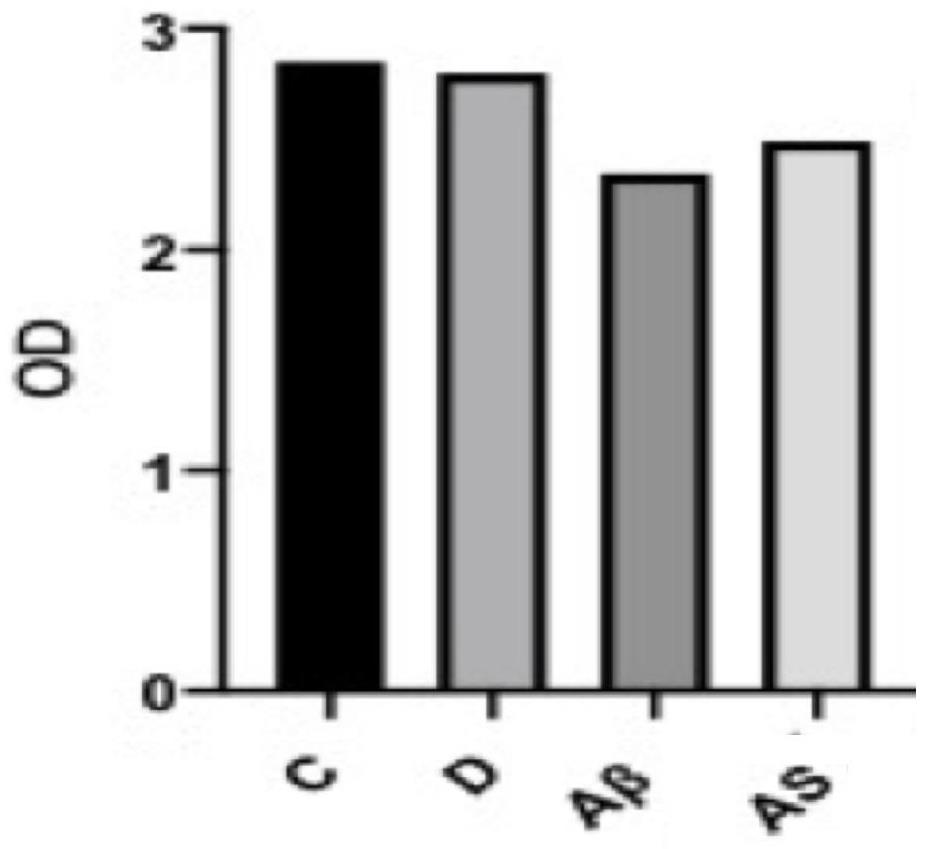
[0032] CCK8 detects primary neuronal viability
[0033] CCK-8 reagent (Cell Counting Kit-8 cell counting reagent) contains WST-8: chemical name: 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl) -5-(2,4-disulfonic acid benzene)-2H-tetrazole monosodium salt, its role in the electron carrier 1-methoxy-5-methylphenazine dimethyl sulfate (1-Methoxy PMS) It is reduced to a highly water-soluble yellow formazan product (Formazan) by the dehydrogenase in the mitochondria of the cell, and the amount of the generated formazan is proportional to the number of living cells. The light absorption value was measured at a wavelength of 450nm by an enzyme-linked immunosorbent assay instrument, which can indirectly reflect the number of living cells. This method has been widely used in the activity detection of some biologically active factors, large-scale antitumor drug screening, cell proliferation test, cytotoxicity test and drug sensitivity test, etc.
[0034] Test materials: 3d SD rats (purc...
Embodiment 2
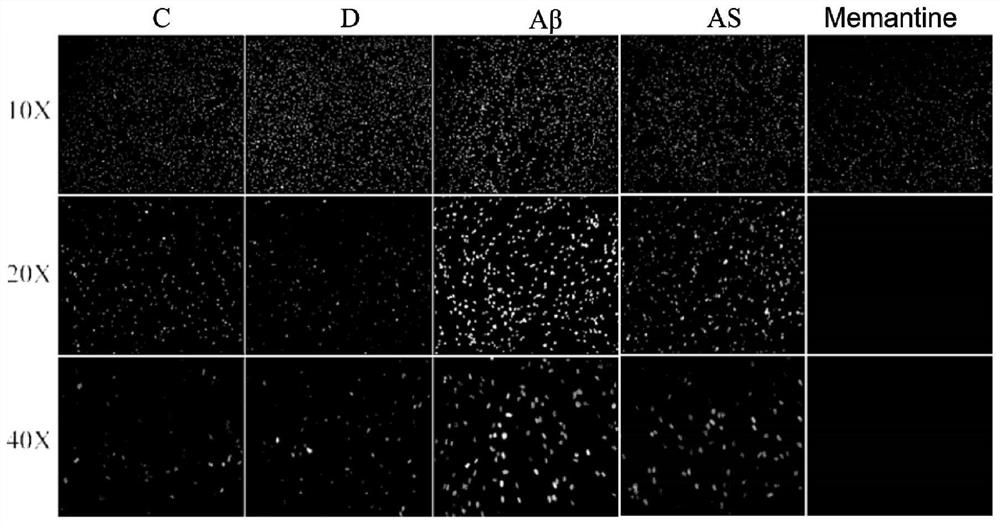
[0040] Immunofluorescence staining
[0041] Immunofluorescence technique (Immunofluorescence technique) is a technique established on the basis of immunology, biochemistry and microscopy techniques. This technique utilizes antigen-antibody reactions to localize antigenic substances in tissues or cells.
[0042] Test materials: 3d SD rats (purchased from Lanzhou University Experimental Animal Center), Anti-MAP2antibody, 488 fluorescent antibody, sheep serum, PBS, fetal bovine serum, horse serum, B27 medium, penicillin and streptomycin, astragaloside.
[0043] The specific experimental steps are as follows:
[0044] Divide the primary neurons into 5 groups, respectively marked as C, D, Aβ, AS, and Memantine, and inoculate them on glass slides in polylysine-coated 24-well plates, and grow the cells to a density of 70%-80% , and completely adhered to the wall, group C did not receive any treatment; group D was treated with a solvent of DMSO:PBS=1:49; group Aβ was added with Aβ ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com