Imidazole derivative and organic electroluminescent device thereof
An imidazole derivative and unsubstituted technology, which is applied in the field of imidazole derivatives and their organic electroluminescent devices, can solve the problems of low luminous efficiency and poor performance, and achieve high luminous efficiency, improved luminous efficiency, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
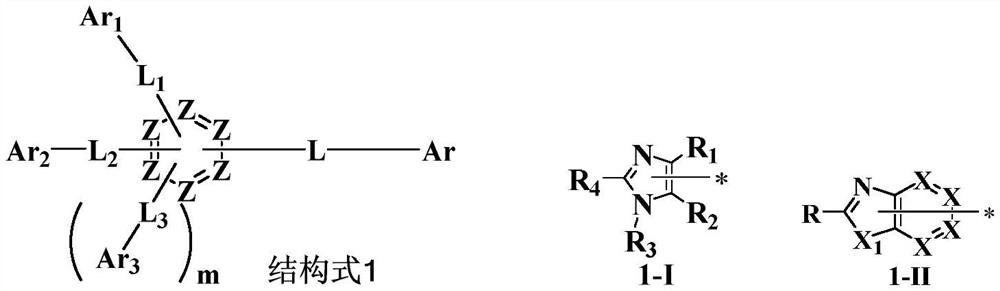
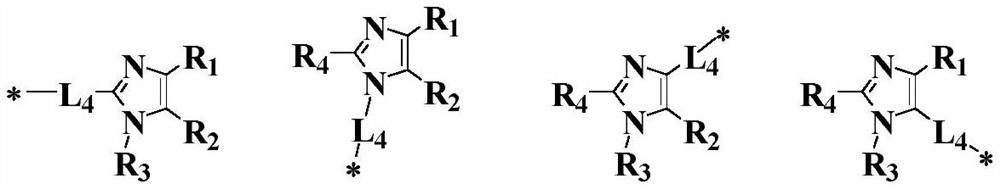
[0133] There is no particular limitation on the preparation method of the imidazole derivative of structural formula 1 in the present invention, and conventional methods well known to those skilled in the art can be used. For example, carbon-carbon coupling reaction, carbon-nitrogen coupling reaction, etc., the imidazole derivatives of structural formula 1 of the present invention can be prepared by the synthetic route shown below.
[0134]
[0135] There is no particular limitation on the preparation method of the diamine derivative of structural formula 2 in the present invention, and conventional methods well known to those skilled in the art can be used. For example, carbon-nitrogen coupling reaction, etc., the diamine derivative of the structure formula 2 of the present invention can be prepared by the synthetic route shown below.
[0136]
[0137] The B m selected from the groups shown below, The Xn is selected from halogens, such as I, Br, Cl and the like.
Embodiment 1
[0367] Embodiment 1: Preparation of organic electroluminescent device 1
[0368] ITO was used as the anode on the glass substrate; m-MTDATA was vacuum-deposited on the anode as a hole injection layer with a thickness of 60nm; NPB was vacuum-deposited in the hole injection layer as a hole transport layer with a thickness of 50nm; vacuum-evaporated CBP:Ir(ppy) on hole transport 3 (10wt%) as the light-emitting layer, the evaporation thickness is 30nm; on the light-emitting layer, the compound 48 of the present invention is vacuum evaporated as the hole blocking layer, and the evaporation thickness is 10nm; on the hole blocking layer, the vacuum evaporation Alq 3 As the electron transport layer, the evaporation thickness is 30nm; on the electron transport layer, Liq is vacuum evaporated as the electron injection layer, and the evaporation thickness is 1nm; on the electron injection layer, Al is vacuum evaporated as the cathode, and the evaporation thickness is 200nm.
[0369] The...
Embodiment 2~30
[0371] Embodiments 2-30: Preparation of Organic Electroluminescent Devices 2-30
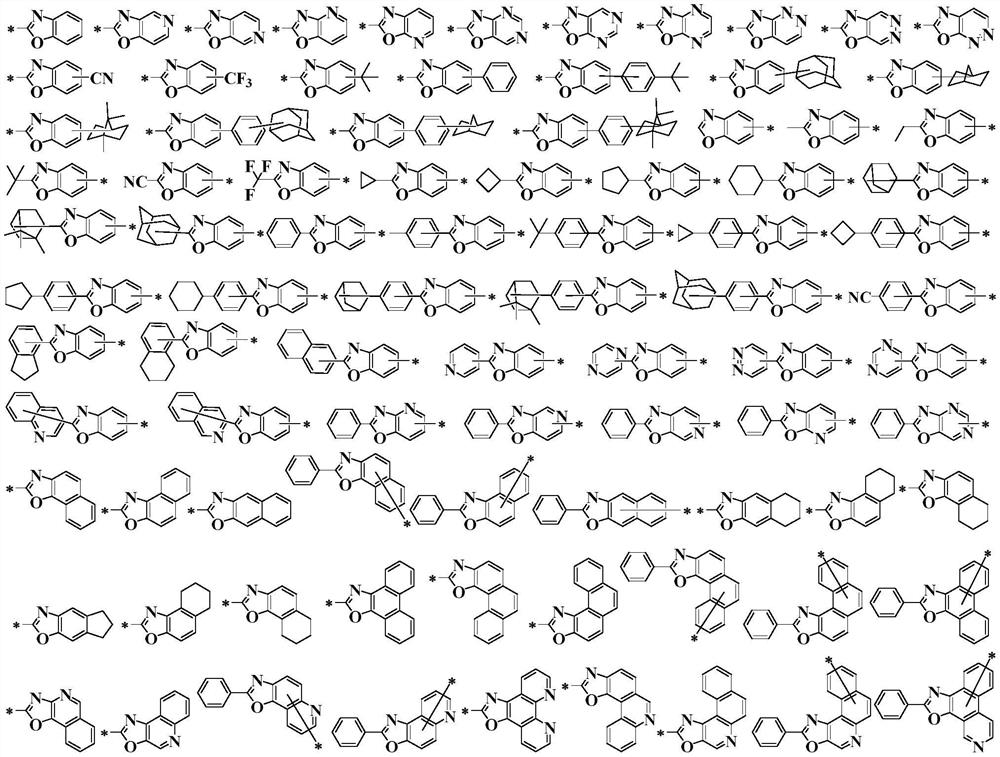
[0372] Compound 48 in the hole blocking layer of Example 1 was replaced by Compound 56, Compound 65, Compound 71, Compound 96, Compound 111, Compound 126, Compound 130, Compound 139, Compound 156, Compound 168, Compound 174, Compound Compound 185, Compound 191, Compound 233, Compound 246, Compound 249, Compound 250, Compound 254, Compound 256, Compound 257, Compound 271, Compound 273, Compound 274, Compound 277, Compound 282, Compound 288, Compound 302, Compound 303 , compound 325, and the other steps were the same to obtain organic electroluminescent devices 2-30.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com