Compound with Janus kinase inhibitory activity, composition comprising compound and application of compound
A kind of kinase inhibition and compound technology, applied in the field of medicine, to achieve the effect of excellent pharmacodynamic performance and excellent Janus kinase inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0079] The preparation of solvates and hydrates is well known in the art, a typical procedure is as follows: Dissolving the compound in the required amount of solvent (organic solvent, water or a mixture of both) at above ambient temperature In this method, the solution is cooled at a rate sufficient to form crystals, the crystals are then isolated by standard methods, and finally the presence of solvent or water in the crystals of the solvate or hydrate is confirmed by analytical techniques (eg, infrared spectroscopy, thermal analysis).
[0080] Since the above compound has excellent Janus kinase inhibitory activity, it can be used as a medicament for preventing and / or treating autoimmune diseases, inflammatory diseases, allergies, cancer, skin diseases, and myelofibrosis.
[0081] In a preferred embodiment of the present invention, the above-mentioned autoimmune diseases are autoimmune skin disorders, multiple sclerosis, rheumatoid arthritis, juvenile arthritis, type I diabet...
Embodiment 1
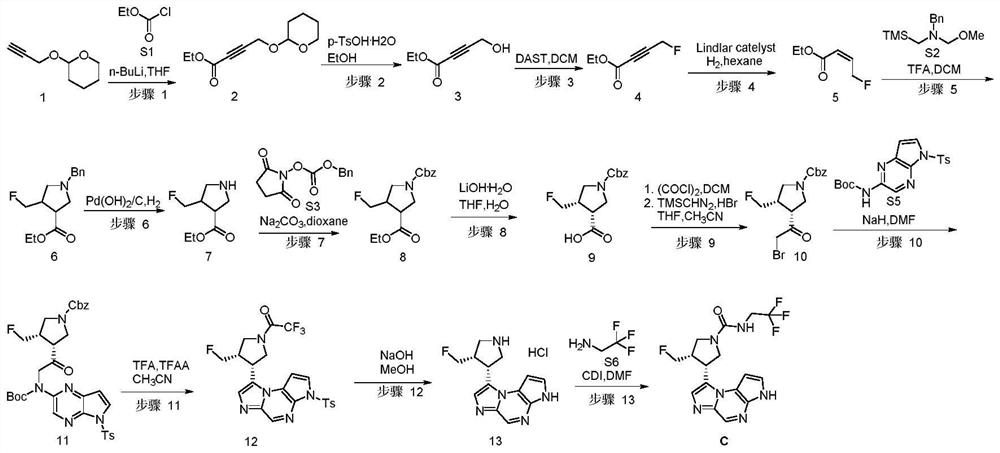
[0097] This example provides the compound (3S,4R)-3-fluoromethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]pyrazine- 8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine-1-amide (C), its preparation process is as follows (wherein the synthetic route of compound C is as follows figure 1 ):
[0098] step 1:
[0099]
[0100] Under nitrogen protection, a solution of compound 1 (10.0 g, 71.3 mmol, 1.0 eq) in anhydrous THF (200 mL) was cooled to -68°C with a dry ice-acetone bath. To the above solution, n-BuLi (29.1 mL, 2.5M in hexane, 72.8 mmol, 1.05 eq) was slowly added dropwise, and the addition was completed in 45 minutes. Subsequently, it was stirred at -65°C for 1 hour. Compound S1 (11.6 g, 107.0 mmol, 1.5 eq) was added dropwise to the above reaction liquid. After dropping, stir at -65°C for 0.5 hours. TLC detection showed that the reaction was complete.
[0101] The reaction solution was quenched with aqueous ammonium chloride (100 mL). Separate the organic phase. The aqueous phase w...
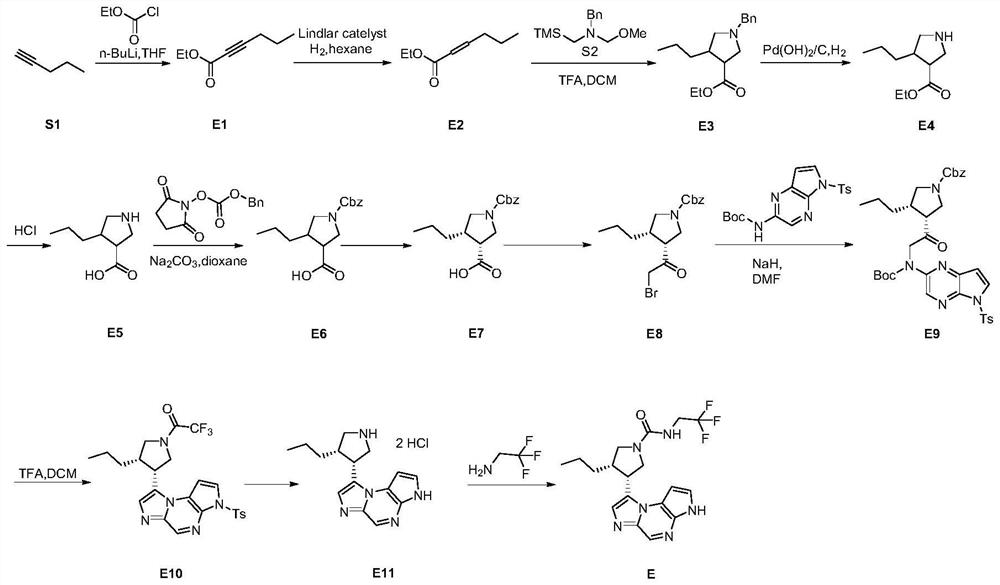
Embodiment 2
[0162] This example provides the compound (3S,4R)-3-ethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]pyrazine-8- Base)-N-(2-fluoro-2-methylpropyl)pyrrolidin-1-amide (D), its preparation process is as follows (such as figure 2 ):
[0163] step 1:
[0164]
[0165] Under nitrogen protection, NaH (0.345 g, 8.61 mmol, 1.0 eq) was slowly added to anhydrous DMF (10 mL), and the ice-salt bath was cooled to 0°C. To the above system, 10 mL of DMF solution of compound a (3.35 g, 8.61 mmol, 1.0 eq) was slowly added dropwise, and the temperature of the addition was controlled not to exceed 0°C. After the addition, the reaction was continued for 30 minutes. Then, 10 mL of DMF solution of compound D-1 (3.05 g, 8.61 mmol, 1.0 eq) was added dropwise to the system, and stirring was continued at 0° C. for 1.5 hours after the addition was complete. TLC showed complete consumption of starting material D-1. The reaction was quenched with 0.2 L of ice water, extracted with ethyl acetate (50 mL×3), a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com