Compound and application thereof
A technology of compounds and microorganisms, applied in organic chemistry, chemical instruments and methods, instruments, etc., can solve problems such as complex chemical structures, tedious synthetic routes, complex purification procedures, etc., and achieve good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
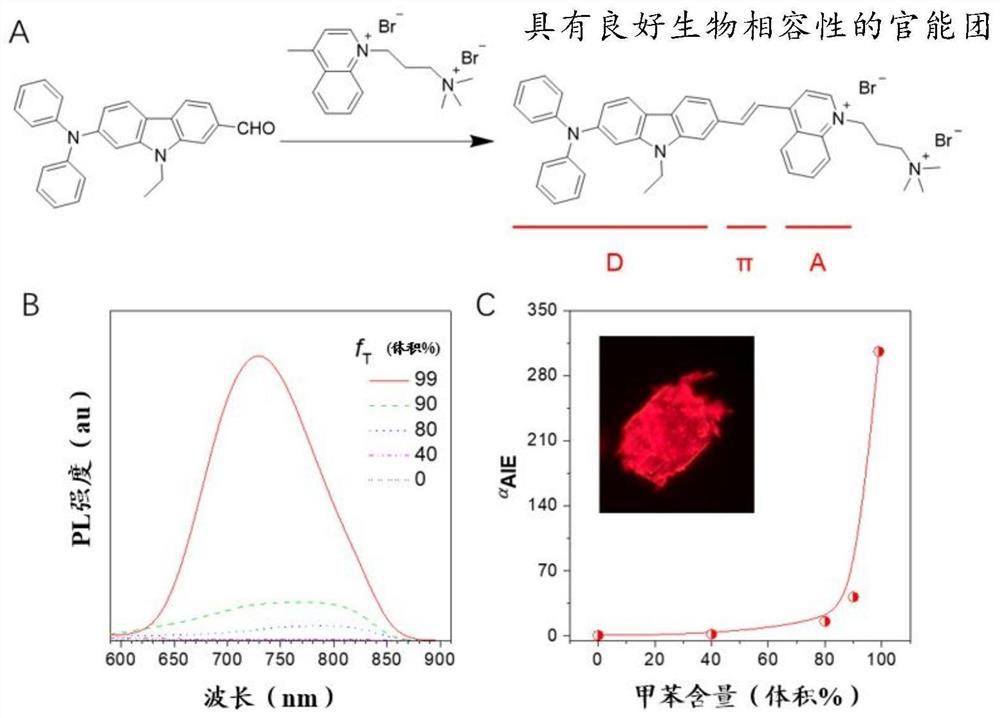
[0112] Embodiment 1: the synthesis of DCQA
[0113] The design principle and synthesis route of DCQA are as follows: figure 1 shown. Specifically, 7-(diphenylamino)-9-ethyl-9H-carbazole-2-carbaldehyde (0.50 g, 1.28 mmol) and 1-(3-trimethylaminopropyl)-4-methanol Quinoline dibromide (0.52 g, 1.28 mmol) was dissolved in absolute ethanol (15 mL). 2 drops of piperidine were added and the solution was refluxed overnight under nitrogen. After cooling to room temperature, the solvent was removed by evaporation under reduced pressure. The residue was purified by column chromatography on neutral alumina, eluting with a dichloromethane / methanol mixture solvent, to afford DCQA as a dark brown crystalline solid. (0.62g, Yield: 62%). 1 H NMR (400MHz, DMSO-d 6 , ppm): δ9.45(d, J=6.5Hz, 1H), 9.17(d, J=8.7Hz, 1H), 8.64-8.59(m, 2H), 8.51-8.41(m, 2H), 8.30- 8.26(m,2H),8.17(d,J=8.1Hz,1H),8.11-8.06(m,2H),7.82(d,J=8.3Hz,1H),7.33-7.29(m,4H),7.18 (s,1H),7.08-7.03(m,6H),6.87(d,J=8.4Hz,1H),5.0...
Embodiment 2
[0118] Embodiment 2: the application of DCQA in microbial staining
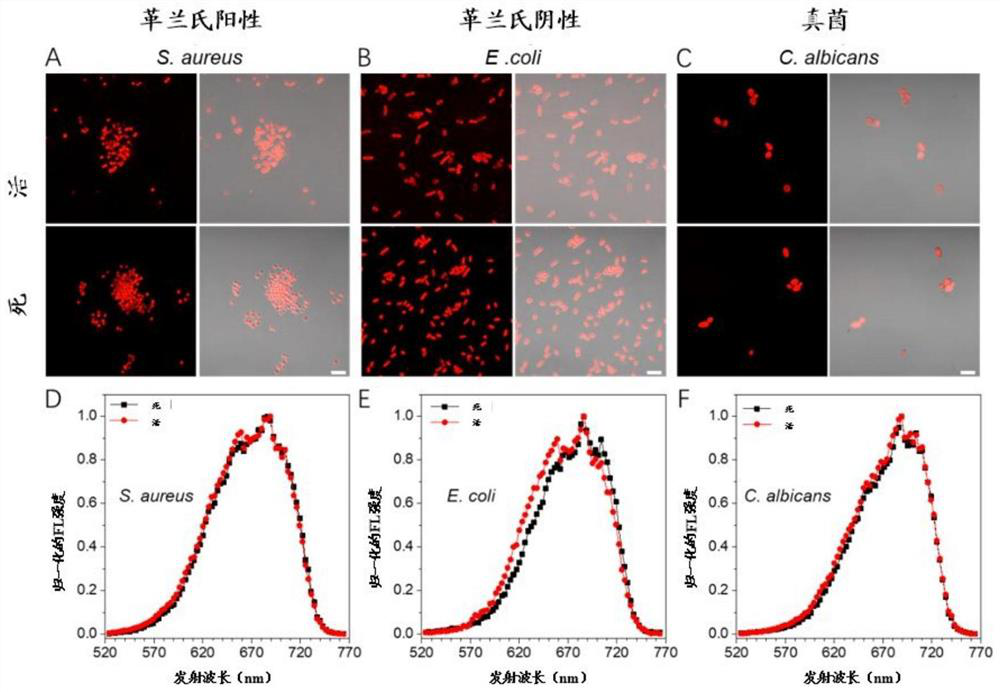
[0119] Microbial imaging experiments were initially performed by incubating DCQA with strains of three indicator types, S. aureus (representing Gram-positive bacteria), E. coli (representing Gram- negative bacteria), C.albicans (representing fungi), followed by CLSM imaging excited at 560nm as figure 2 A-2C shown. Depend on figure 2 It can be seen from A-2C that all three microorganisms are highly stained by DCQA.
[0120] In addition, DCQA showed good staining ability in both live and dead states. The incubation time test showed that DCQA can interact with various microorganisms in a short time, and a strong signal can be detected in only 5 minutes, such as Figure 8shown. Afterwards, the fluorescence signal did not change much, indicating that DCQA has a suitable staining window for both imaging and high-throughput analysis. This proves that DCQA can be used as a general detection probe for various ...
Embodiment 3
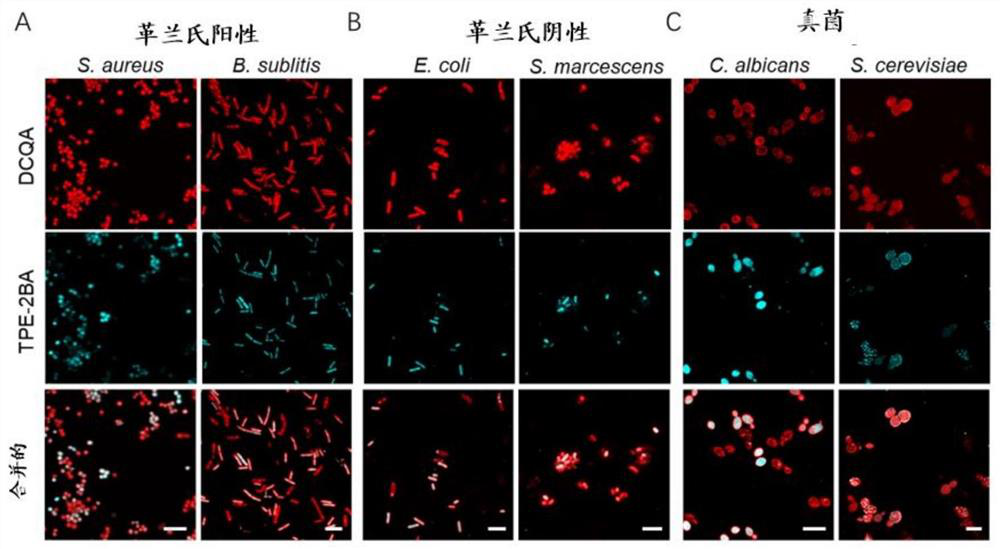
[0123] Example 3: Application of two-component aggregation-induced luminescent fluorescent luminescent agent system in monitoring the metabolic state of microorganisms
[0124] The metabolic state of microorganisms is closely related to their functions, biofilm formation ability, virulence factor secretion, drug resistance generation and other characteristics. As the environment changes, the metabolic state of microorganisms also changes. Especially for opportunistic pathogens, changes in their metabolic levels can lead to situations that are harmful to us. Microbial activity is a key factor in microbial metabolism. When confronted with certain antibiotics, some bacteria reportedly sacrifice themselves to warn other bacteria of the danger by spreading out their resistance genes. The remaining microbial species will pick up resistance genes and acquire resistance to specific antibiotics directly. Therefore, it is very important to monitor the metabolic state of microorganism...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com