Infusion cell freezing medium and application thereof
A cryopreservation solution and cell technology, applied in the field of cell and cell engineering, can solve the problems of allergy and unstable cell cryopreservation solution, and achieve the effects of high safety, good viability and killing ability, and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1 can infuse cell cryopreservation solution
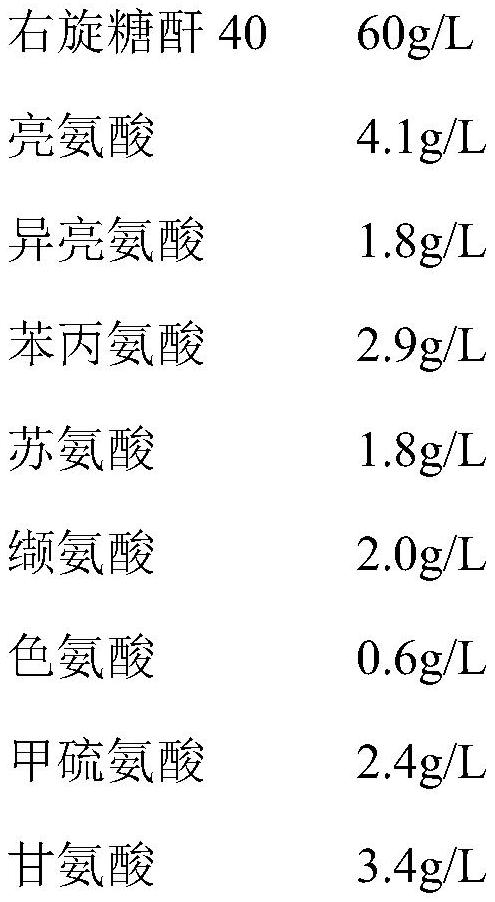
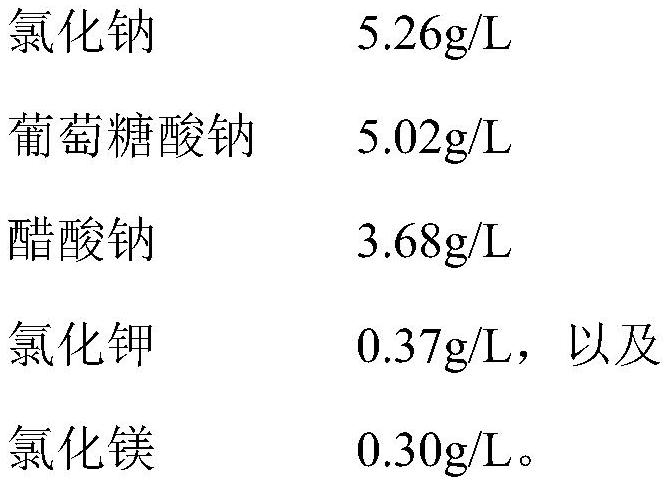
[0031] This embodiment provides an infusionable cell cryopreservation solution, the components of which include by volume percentage: glycerin 10v / v%, human albumin injection 20v / v%, hydroxyethyl starch 40 sodium chloride injection 62.2 v / v%, dextran 40 amino acid injection 3.6v / v%, BCG polysaccharide nucleic acid injection 0.5v / v%, and compound electrolyte injection 3.7v / v%.
Embodiment 2
[0032] Embodiment 2 can infuse cell cryopreservation solution
[0033] This embodiment provides an infusible cell cryopreservation solution, the components of which include by volume percentage: glycerin 10v / v%, human albumin injection 20v / v%, and hydroxyethyl starch 40 sodium chloride injection 70v / v%.
Embodiment 3
[0034] Embodiment 3 can transfuse cell cryopreservation solution
[0035] This embodiment provides an infusionable cell cryopreservation solution, the components of which include by volume percentage: glycerin 10v / v%, human albumin injection 20v / v%, hydroxyethyl starch 40% and sodium chloride injection 60v / v%, and BCG polysaccharide nucleic acid injection 10v / v%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com