N-aryl pyridine thiazolothiazole-cucurbituril compound as well as preparation method and application thereof
A technology of arylpyridinethiazole and cucurbituril, which is applied in the field of nanozymes, to achieve precise controllability, good application prospects, and avoid background interference and biological body damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] A preparation method of N-arylpyridinethiazolothiazole-cucurbituril complex, comprising the steps of:
[0050] S1. According to the report of the document "Thiazolothiazole Fluorophores ExhibitingStrong Fluorescence and Viologen-Like Reversible Electrochromism" published on JACS, synthesize 2,5-bis(4-pyridyl)thiazolothiazole, the 2,5-bis(4-pyridine base) thiazolothiazole as an intermediate, which is dissolved in 2,4-dinitrofluorobenzene, and 2,5-bis(4-pyridyl)thiazolothiazole and 2,4-dinitrofluoro The molar ratio of benzene is 1:(42-85), then heated at 90°C for 48 h, cooled, washed three times with n-hexane, and vacuum-dried to obtain Zincke salt, that is, N-aryl or N-alkylpyridinium salt;
[0051] S2. the Zincke salt obtained in step S1 and p-chloroaniline are placed in a round-bottomed flask according to a molar ratio of 1:(2-4), add 20 mL of ethanol and water and mix, wherein the volume ratio of ethanol and water is 3 : 2, then heated to reflux at 100°C for 48 h, af...
Embodiment 1
[0053] Example 1, 0.126 g of Zincke salt, 0.153 g of p-chloroaniline, 12 mL of ethanol and 8 mL of water were placed in a 100 mL round bottom flask. The reaction mixture was refluxed at 100 °C for 48 h. After the reaction was completed, it was washed with ether, and the obtained crude product was recrystallized in methanol solution to obtain pure grass-green N-arylpyridinium salt (38.6 mg, 33.7% yield).
[0054] 1 H NMR (600 MHz, D 2 O): δ 9.19 (d, J = 6.4 Hz, 4H), 8.75 (d, J = 6.6Hz, 4H), 7.73 (d, J = 2.8 Hz, 8H).
[0055] 0.126 g of Zincke's salt, 0.0765 g of p-chloroaniline, 12 mL of ethanol and 8 mL of water were placed in a 100 mL round bottom flask. The reaction mixture was refluxed at 100 °C for 48 h. After the reaction is completed, wash with ether, and recrystallize the obtained crude product in a methanol solution to obtain a pure grass-green N-arylpyridinethiazolothiazole molecule.
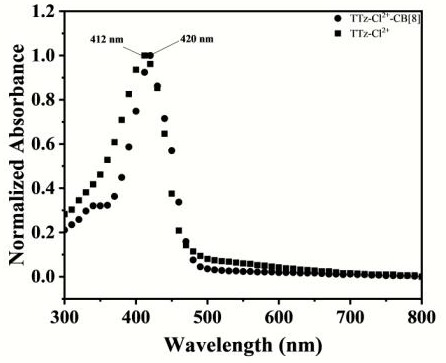
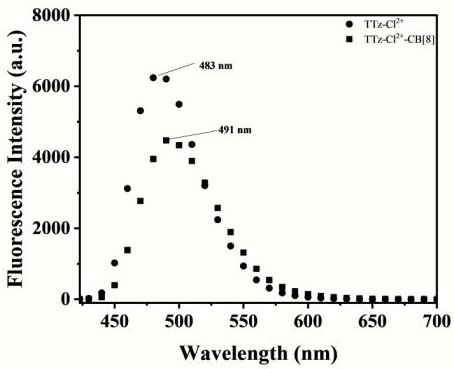
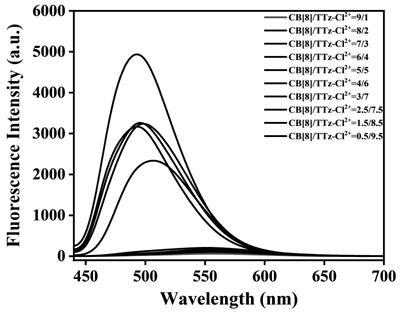
[0056] N-arylpyridinethiazolothiazole molecules and cucurbituril 8 were respec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com