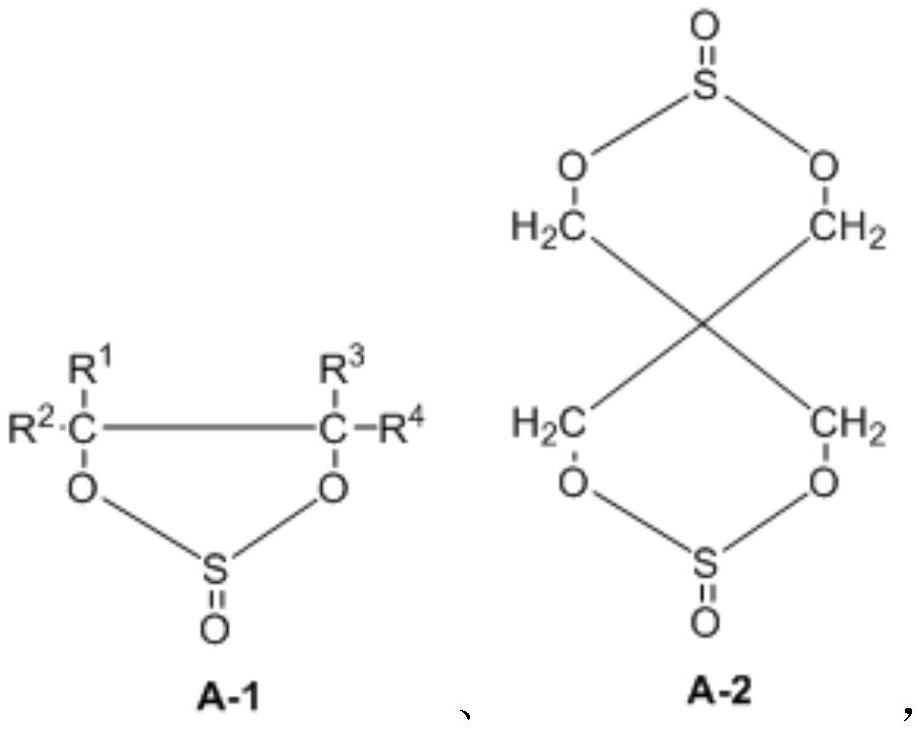
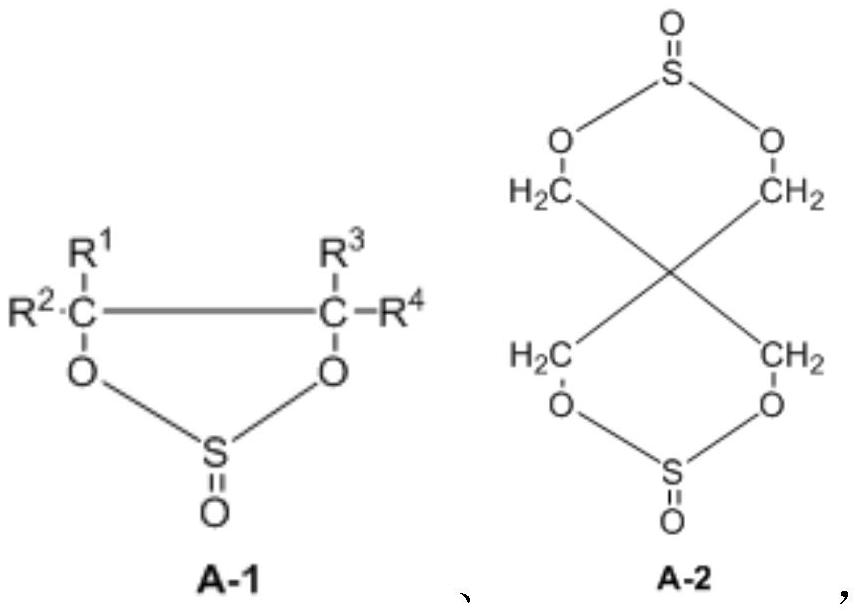
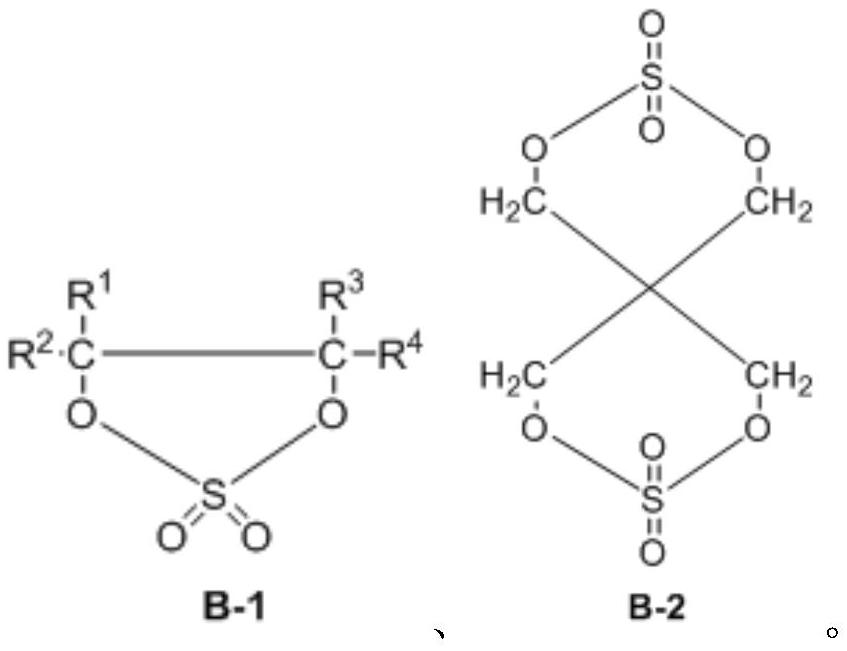
Synthesis method of cyclic sulfate
A technology of cyclic sulfate and cyclic sulfite, which is applied in the field of preparation of cyclic sulfate, can solve the problems of cumbersome catalyst recovery, expensive catalyst, and a lot of waste water, and achieve the effect of low cost, low price and simple separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Under oxygen atmosphere (20L oxygen bag, normal pressure), add Fe(NO 3 ) 3 9H 2 O (406mg, 1mmol), 4-OH-TEMPO (342mg, 2mmol), NaCl (118mg, 2mmol), pentaerythritol bicyclic sulfite (2.28g, 10mmol), 40ml of dichloroethane solvent, stirred the reaction at 50°C 12 hours to end the reaction, transfer to a separatory funnel, add 50 ml of water, fully wash, the organic phase is dried with anhydrous sodium sulfate and distilled under reduced pressure to recover dichloroethane, and the residue is subjected to silica gel column chromatography, ethyl acetate and petroleum Ether was used as the mobile phase, the solvent was removed, and vacuum-dried to obtain 2.34 g of pentaerythritol bicyclic sulfate as a white solid, with a yield of 90% and a gas-phase purity of 99%. 1 H NMR (400MHz, CD 3 COCD 3 )δ5.02(s,8H).
Embodiment 2
[0021] Add Fe(NO 3 ) 3 9H 2 O (8g, 20mmol), 4-OH-TEMPO (6.84g, 40mmol), NaCl (2.36g, 40mmol), 32.4 grams of vinyl sulfite (0.3 moles), 900 milliliters of dichloroethane solvent, at 100ml / min Continue to feed air at a constant flow rate, stir and react at 30°C for 6 hours to end the reaction, transfer to a separatory funnel, add 1000 ml of water, wash thoroughly, dry the organic phase with anhydrous sodium sulfate, and distill under reduced pressure to recover dichloroethane, and the remaining The product was vacuum-dried to obtain a crude vinyl sulfate product, which was recrystallized with dichloromethane to obtain 26 g of white crystalline vinyl sulfate, with a yield of 70%, a melting range of 98-99° C., and a gas-phase purity of 99%.
Embodiment 3
[0023] In the autoclave, 4-methyl vinyl sulfite (0.3 mol) was dissolved in 900 ml of toluene, kept at 45 ° C, and iron sulfate (20 mmol), TEMPO (40 mmol), NaCl (2.36 g, 40 mmol) were added successively under stirring, In an oxygen atmosphere of 5 atmospheres, stir and react for 10 hours, separate the organic phase and dry it with anhydrous sodium sulfate, and carry out vacuum distillation (50°C / 1mmHg) after the filtrate is precipitated to obtain 29.8 grams of transparent liquid 4-methyl Vinyl sulfate, yield 72%, gas phase purity 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com