Para-aryl substituted pyridinium salt derivative electrochromic material and preparation method thereof
A technology of electrochromic materials and derivatives, applied in the direction of color-changing fluorescent materials, chemical instruments and methods, organic chemistry, etc., can solve the problems of high mortality and human toxicity, and achieve simple synthesis, low toxicity and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] A preparation method of a para-aryl-substituted pyridinium salt derivative electrochromic material 1-benzyl-4-phenylpyridinium bromide, comprising the following steps:
[0049] 1) Mix pyridine 4-boronate, chlorobenzene, sodium hydroxide, palladium chloride, tri-tert-butylphosphine (molar ratio 1:1:8:0.5:2) and water evenly, and place in N 2 Stir at room temperature under atmosphere for 20-30min, and reflux at 100°C for 14h; after the reaction is completed, cool the solution to room temperature, filter, and concentrate the filtrate; recrystallize with ethyl acetate to obtain 4-phenylpyridine;
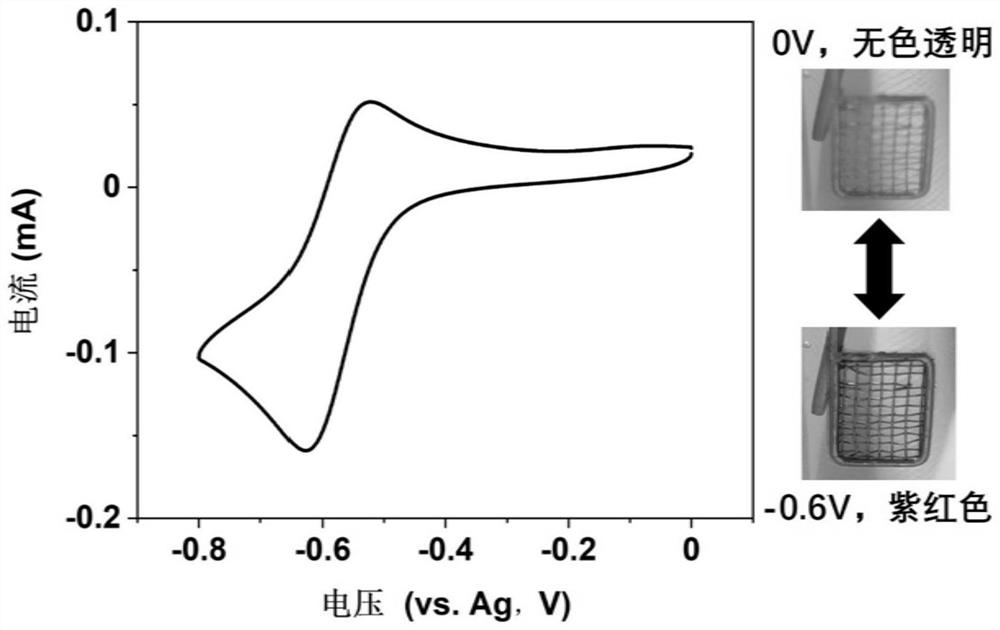
[0050] 2) Mix 4-phenylpyridine, benzyl bromide (molar ratio 1:2) and DMF obtained in 1) evenly, reflux at 80-85°C for 0.1h, and remove the solvent by rotary evaporation; recrystallize with dichloromethane to obtain the molecular structure formula 1-benzyl-4-phenylpyridinium bromide salt electrochromic material, its 1 H NMR spectrum as figure 1 shown. The overall yield for the t...
Embodiment 2
[0054] A preparation method of an electrochromic material 1-benzyl-4-(3-(trifluoromethyl)phenyl)pyridinium bromide salt substituted by a para-aryl group, comprising the steps of:
[0055] 1) Mix 4-pyridine boronic acid, 3-(trifluoromethyl)bromobenzene, cesium fluoride, tridibenzylideneacetone palladium (molar ratio 1:2:4:0.01) and DMF evenly, and place in N 2 Stir at room temperature under atmosphere for 20-30min, reflux at 120°C for 70h; after the reaction is complete, cool the solution to room temperature, filter, and concentrate the filtrate; recrystallize with ethyl acetate to obtain 4-(3-(trifluoromethyl)phenyl) pyridine;
[0056] 2) Mix 4-(3-(trifluoromethyl)phenyl)pyridine, benzyl bromide (molar ratio 1:2.5) and DMF obtained in 1) evenly, reflux at 80-85°C for 2 hours, and remove the solvent by rotary evaporation; Obtain molecular structure formula 1-benzyl-4-phenylpyridinium bromide electrochromic material with dichloromethane recrystallization, its 1 H NMR spectrum ...
Embodiment 3
[0060] A preparation method of an electrochromic material 1-benzyl-4-(3-thiophene) pyridinium bromide salt substituted by a para-aryl group, comprising the steps of:
[0061] 1) Mix 4-chloropyridine, 3-(boronic acid) thiophene, potassium tert-butyl, bis(tri-tert-butyl)palladium, triphenylphosphine (molar ratio 1:1.5:5:0.025:0.5) and dioxygen Hexacyclic mixed well, in N 2 Under atmosphere, stir at room temperature for 20-30 minutes, and reflux at 100°C for 46 hours; after the reaction is completed, cool the solution to room temperature, filter, and concentrate the filtrate; recrystallize with ethyl acetate to obtain 4-(3-thiophene)pyridine;
[0062] 2) Mix 4-(3-thiophene)pyridine, benzyl bromide (molar ratio 1:2) and diethyl ether obtained in 1) evenly, reflux at 80-85°C for 2 hours, and remove the solvent by rotary evaporation; recrystallize with dichloromethane to obtain Molecular structural formula 1-benzyl-4-(3-thiophene) pyridinium bromide electrochromic material, its 1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com