Near-infrared fluorescent molecular probe as well as preparation method and application thereof
A fluorescent molecular probe and near-infrared technology, applied in fluorescence/phosphorescence, chemical instruments and methods, and material analysis through optical means, can solve problems such as small changes in fluorescent signals, weak biological penetration, and unfavorable image analysis , to achieve high yield, wide detection range and low fluorescence background signal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
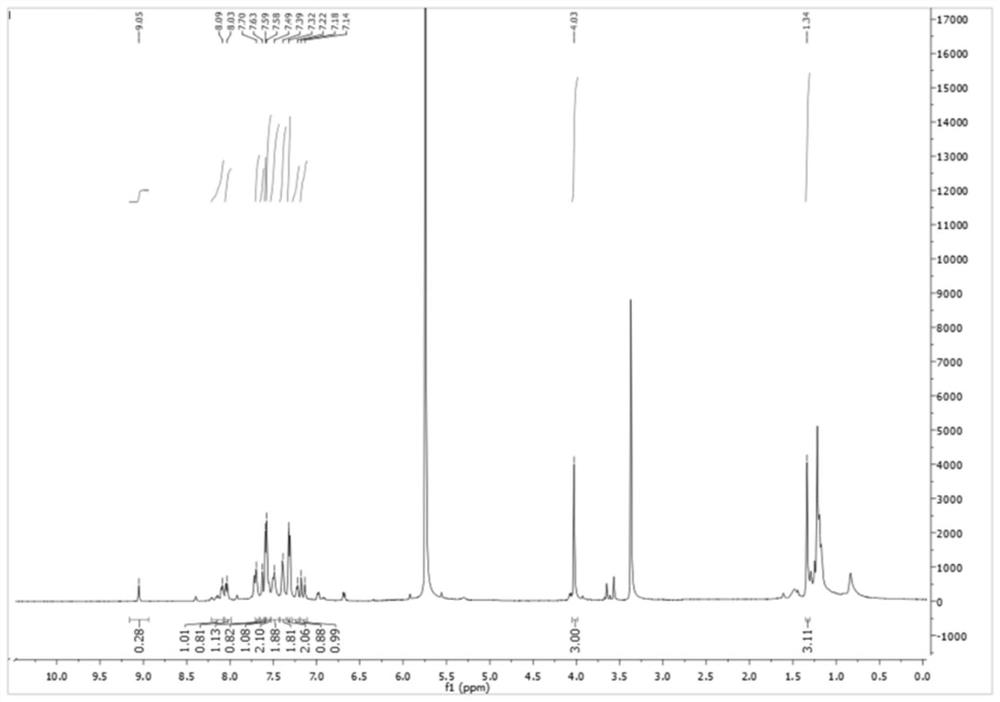
[0046] Synthesis of Fluorescent Probe II
[0047] R in the fluorescent probe structural formula prepared in this embodiment 1 = H, R 2 =H.
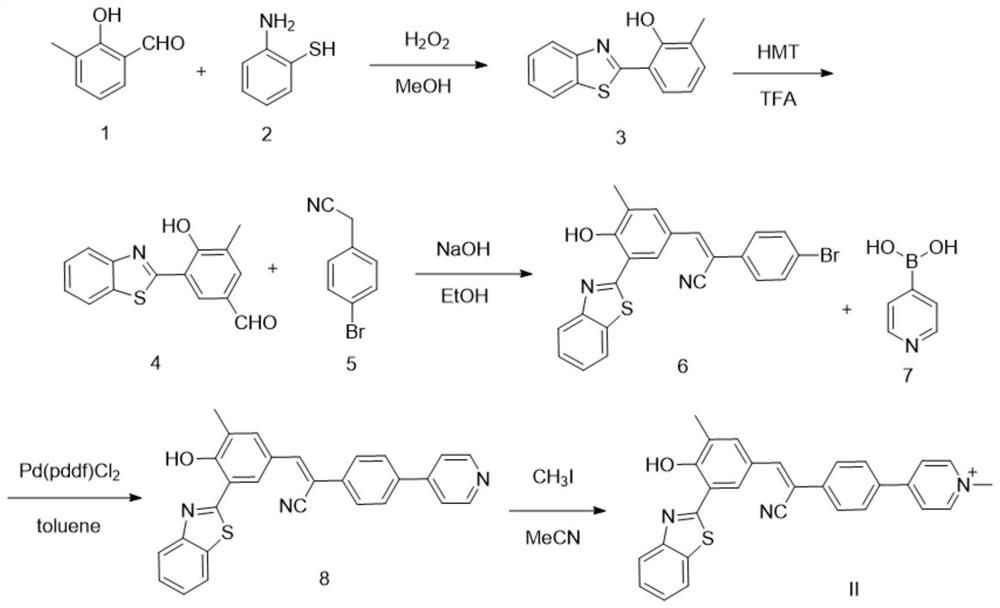
[0048] The synthesis technique route of present embodiment is as figure 1 As shown, it specifically includes the following steps:
[0049] Synthesis of compound 3
[0050] Weigh compound 1 (1.96g, 10mmol) and place it in a 250mL round-bottomed flask, add 40mL of methanol, and after dissolving, drop in compound 2 (1.25g, 10mmol) under stirring at room temperature, and stir at room temperature for 5h. During the reaction, the reaction solution gradually From gray turbid liquid to light yellow clear liquid; TLC monitors the reaction process, the dark spots of raw materials gradually decrease, and blue fluorescent spots with relatively less polarity appear. After the reaction was completed, the reaction solution was poured into 200 mL of water, and a large amount of light yellow solid precipitated out. The light yellow solid was obtained...
Embodiment 2
[0061] Synthesis of Fluorescent Probe III
[0062] R in the fluorescent probe structural formula prepared in this embodiment 1 =CH 3 , R 2 =H.
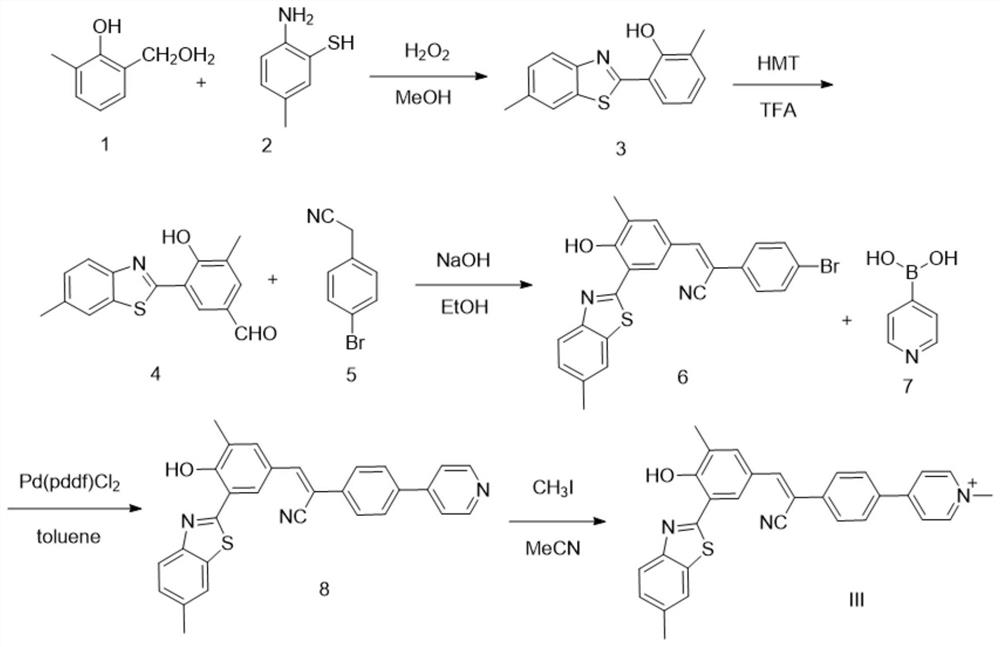
[0063] The synthesis technique route of present embodiment is as figure 2 As shown, the specific steps include:
[0064] Synthesis of compound 3
[0065] Weigh compound 1 (1.96g, 10mmol) and place it in a 250mL round-bottomed flask, add 40mL of methanol, and after dissolving, drop in compound 2 (1.40g, 10mmol) under stirring at room temperature, and stir at room temperature for 5h. During the reaction, the reaction solution gradually From gray turbid liquid to light yellow clear liquid; TLC monitors the reaction process, the dark spots of raw materials gradually decrease, and blue fluorescent spots with relatively less polarity appear. After the reaction was completed, the reaction solution was poured into 200 mL of water, and a large amount of light yellow solid precipitated out. The light yellow solid was obtained by suction ...
Embodiment 3
[0075] Synthesis of Fluorescent Probe IV
[0076] R in the fluorescent probe structural formula prepared in this embodiment 1 =CH 3 , R 2 =CH 3 .
[0077] The synthesis technique route of present embodiment is as Figure 4 As shown, the specific steps include:
[0078] Synthesis of compound 3
[0079]Weigh compound 1 (1.96g, 10mmol) and place it in a 250mL round-bottomed flask, add 40mL of methanol, and after dissolving, drop in compound 2 (1.40g, 10mmol) under stirring at room temperature, and stir at room temperature for 5h. During the reaction, the reaction solution gradually From gray turbid liquid to light yellow clear liquid; TLC monitors the reaction process, the dark spots of raw materials gradually decrease, and blue fluorescent spots with relatively less polarity appear. After the reaction was completed, the reaction solution was poured into 200 mL of water, and a large amount of light yellow solid precipitated out. The light yellow solid was obtained by sucti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com