N-substituted thiazolidone compound and application thereof
A technology of thiazolidinone and compound, applied in the field of medicinal chemistry, to achieve the effect of great development and application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The preparation method of the compound of the above formula (I) is to use phenyl isothiocyanate as the starting material, react with methoxypropylamine to generate substituted phenylthiourea, and then undergo a ring-closing reaction with ethyl chloroacetate to form The N-substituted thiazole heterocyclic ring is finally condensed with substituted benzaldehyde to obtain the final product, and the specific steps are shown in Example 1.
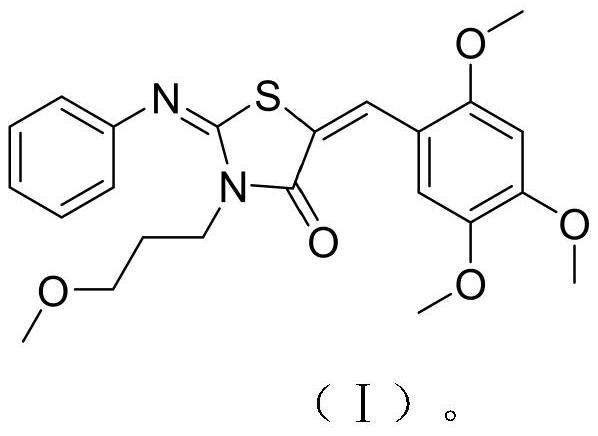
[0027] The N-substituted thiazolidinone compound of the present invention can be used as an aromatic hydrocarbon receptor antagonist, wherein the compound is 2-phenylimino-N-(3-methoxypropyl)-5-(2,4 ,5-trimethoxybenzylidene)thiazolidinone.
[0028] In order to better understand the essence of the present invention and the effects of the compounds described in the invention, the following combined with the experiments and results of the MPTO compound further elaborates its application as an aromatic hydrocarbon receptor antagonist in block...
Embodiment 1
[0037] The preparation of 2-phenylimino-N-(3-methoxypropyl)-5-(2,4,5-trimethoxybenzylidene)thiazolidinone, the synthetic route is shown in (Ⅱ):
[0038]
[0039] [1] Dissolve 6.75g (50mmol) of phenyl isothiocyanate in 25mL of methanol solution, add 5.34g (60mmol) of 3-methoxypropylamine, and heat to 80°C under reflux with stirring, the mixture becomes clear, and the reaction 12 After one hour, thin-layer chromatography (TLC) monitors reaction, and after reaction finishes, mixture is cooled to room temperature, and viscous oily liquid appears, extracts with ethyl acetate, and extracting solution is with 10% hydrochloric acid solution, sodium chloride saturated solution, The deionized water was washed successively, and the solvent was distilled off from the extract under reduced pressure to obtain N-(3-methoxypropyl)phenylthiourea.
[0040] [2] Dissolve 1.34g (6.0mmol) of N-(3-methoxypropyl)phenylthiourea and 2.48g (30mmol) of anhydrous sodium acetate in 20mL of ethanol, and ...
Embodiment 2
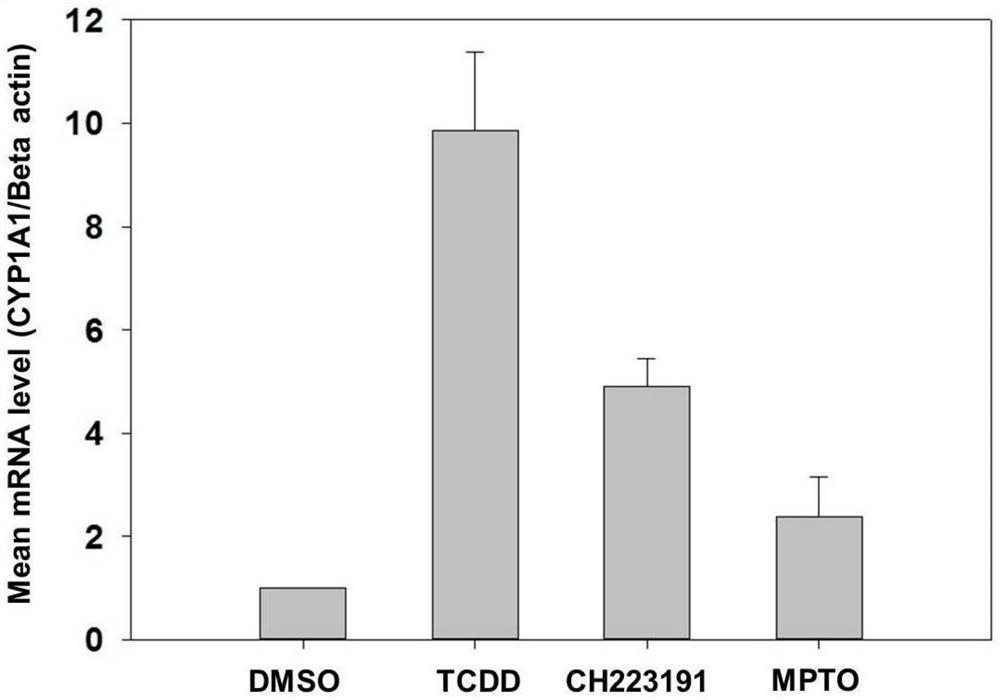
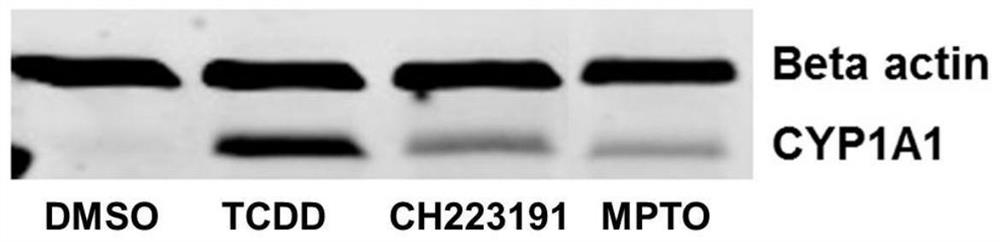
[0045] 2-phenylimino-N-(3-methoxypropyl)-5-(2,4,5-trimethoxybenzylidene)thiazolidinone compound inhibits CYP1A1 gene expression caused by TCDD (1nmol) raised.
[0046] 1. Dilute HepaWT cells at 1×10 4 / mL density to inoculate cell culture dishes with a diameter of 60 mm. After 24h, first add 2-phenylimino-N-(3-methoxypropyl)-5-(2,4,5-trimethoxybenzylidene)thiazolidinone (10μmol), CH223191 (10μmol , classic AhR small molecule inhibitor) were added to two petri dishes respectively, 1nmol of TCDD was added after 1 hour, DMSO was added to the other two petri dishes as a negative control and TCDD (1nmol) was used as a positive control. %CO 2 Under the conditions of the role of 24h.
[0047] 2. Extraction of RNA: Collect the cells, discard the medium, and rinse twice with preheated PBS solution. Add an appropriate amount of lysis buffer to lyse the cells, and pipette slowly several times. The lysate was transferred to a spin column placed in a 2 mL collection tube and centrifu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com